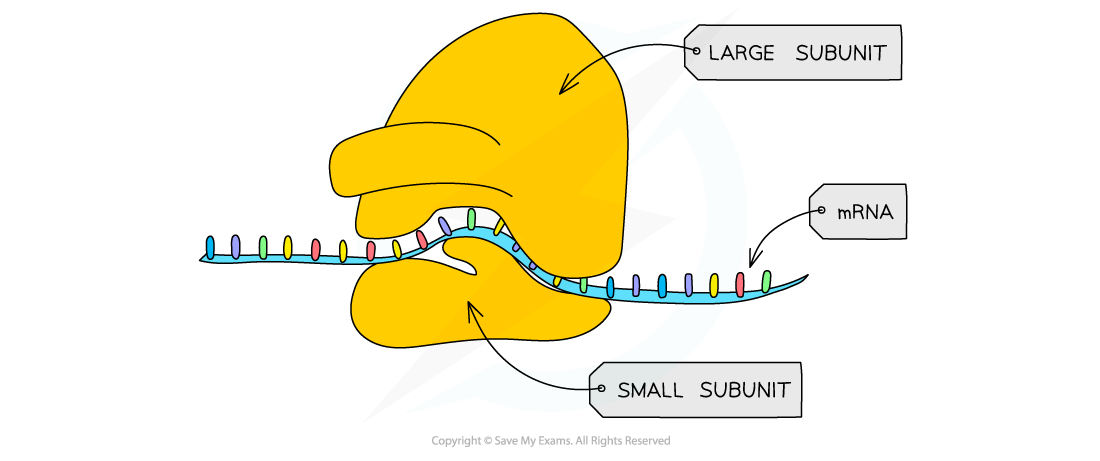
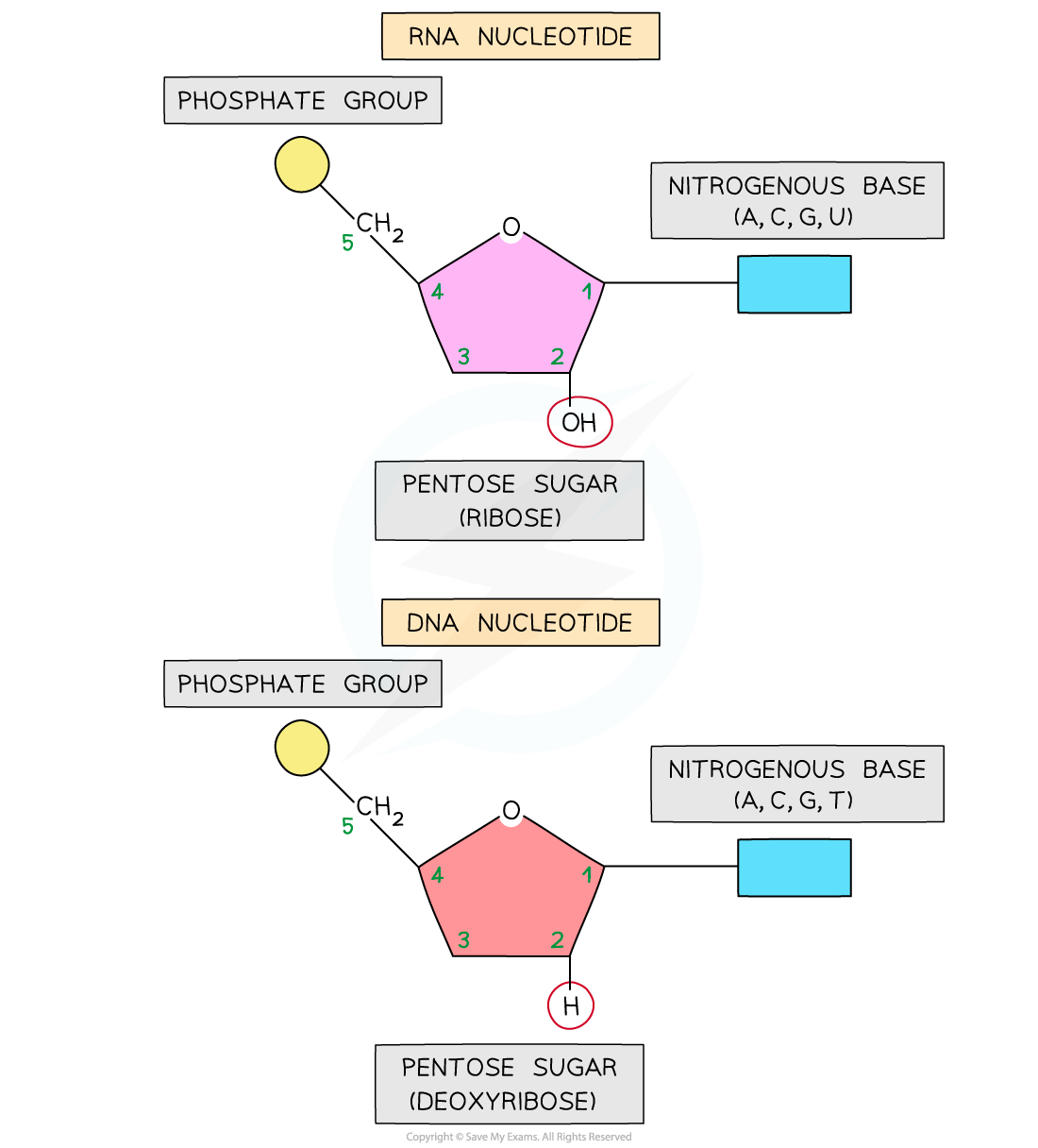
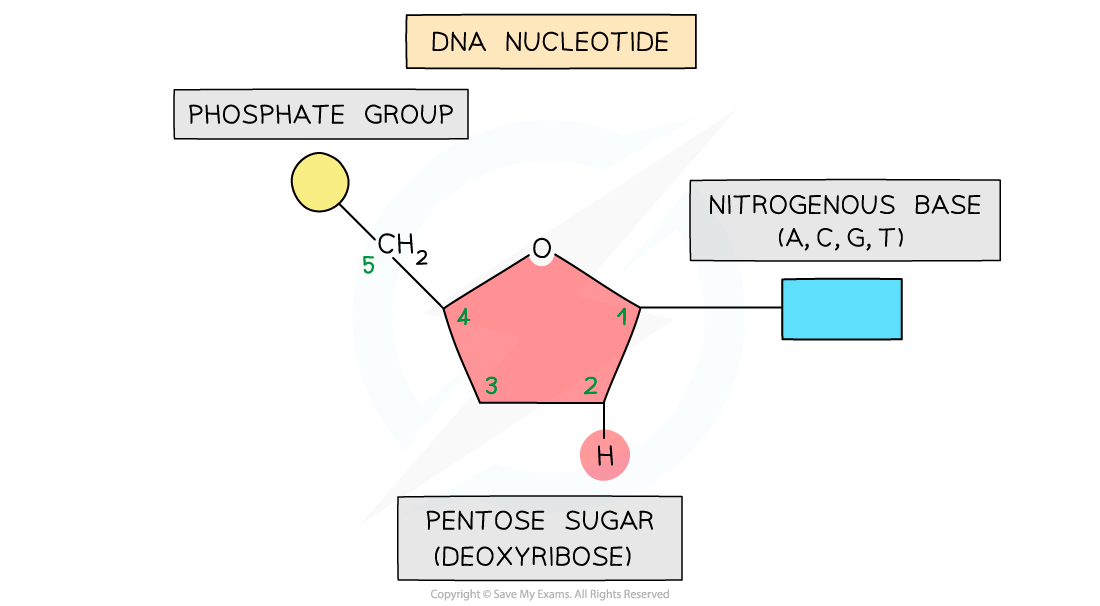
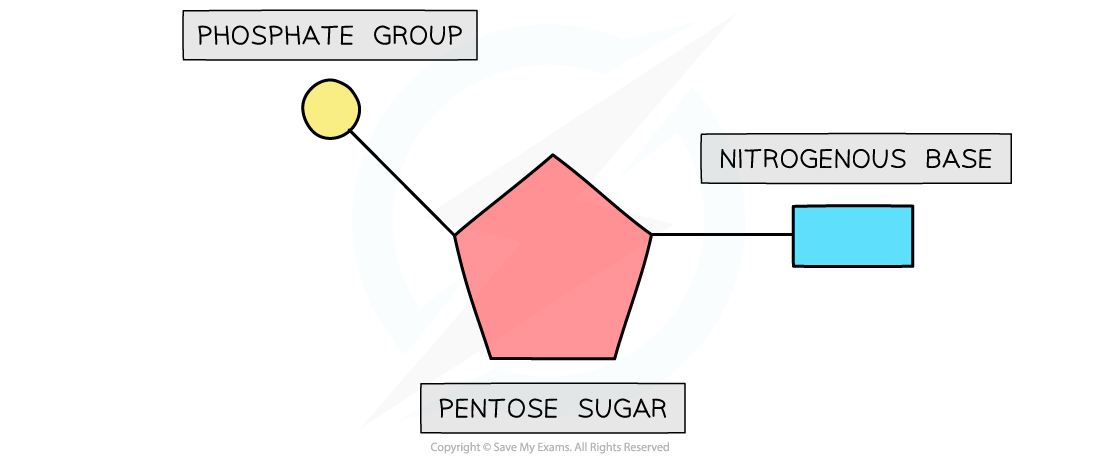
AQA A Level Biology复习笔记 1.5.6 The Origins of Research on the Genetic Code
Appreciating How the Function of DNA Was Determined DNA was actually first observed in the 1800s by a Swiss scientist called Friedrich Miescher Miescher is credited with being the first pers...