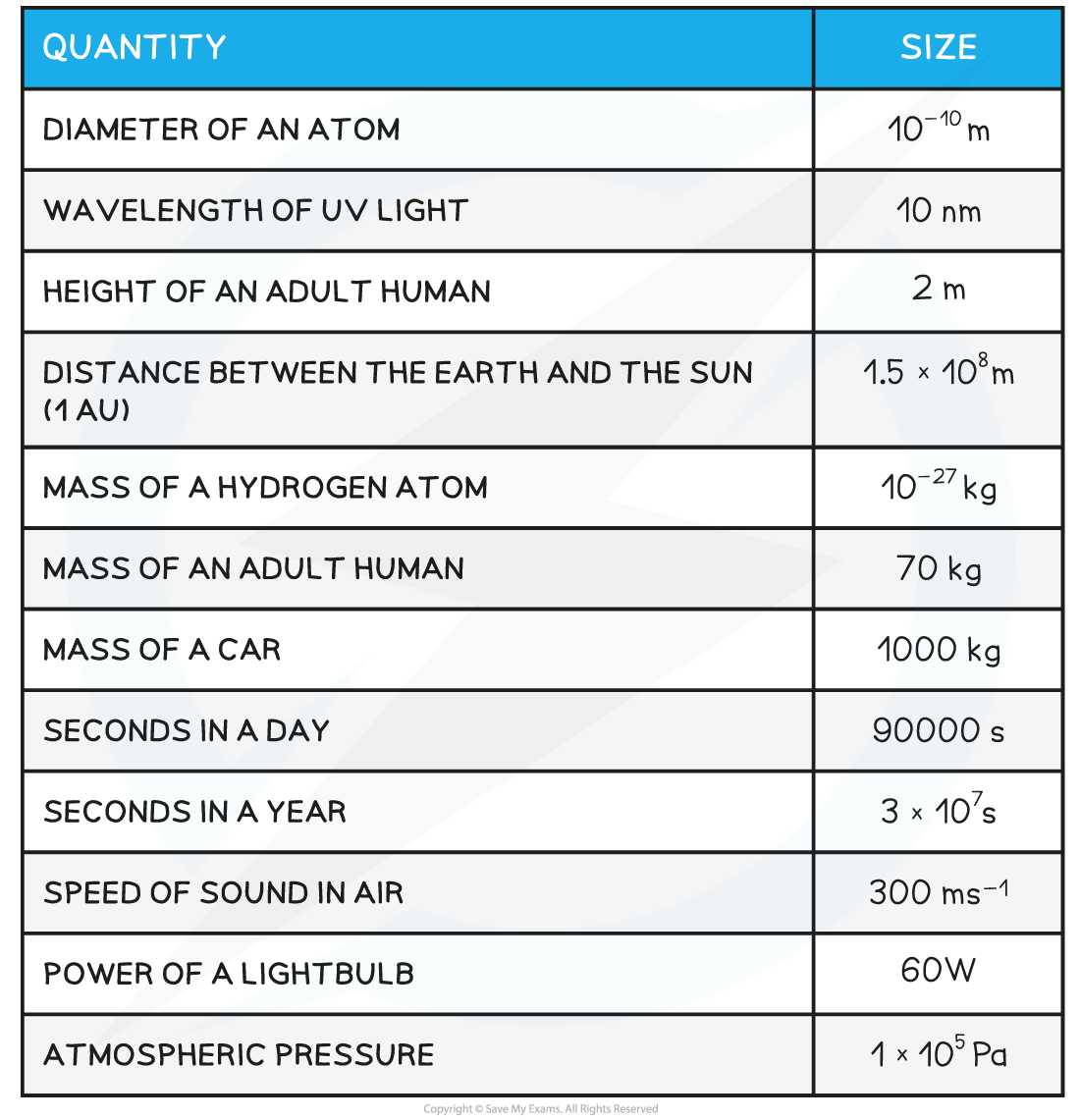
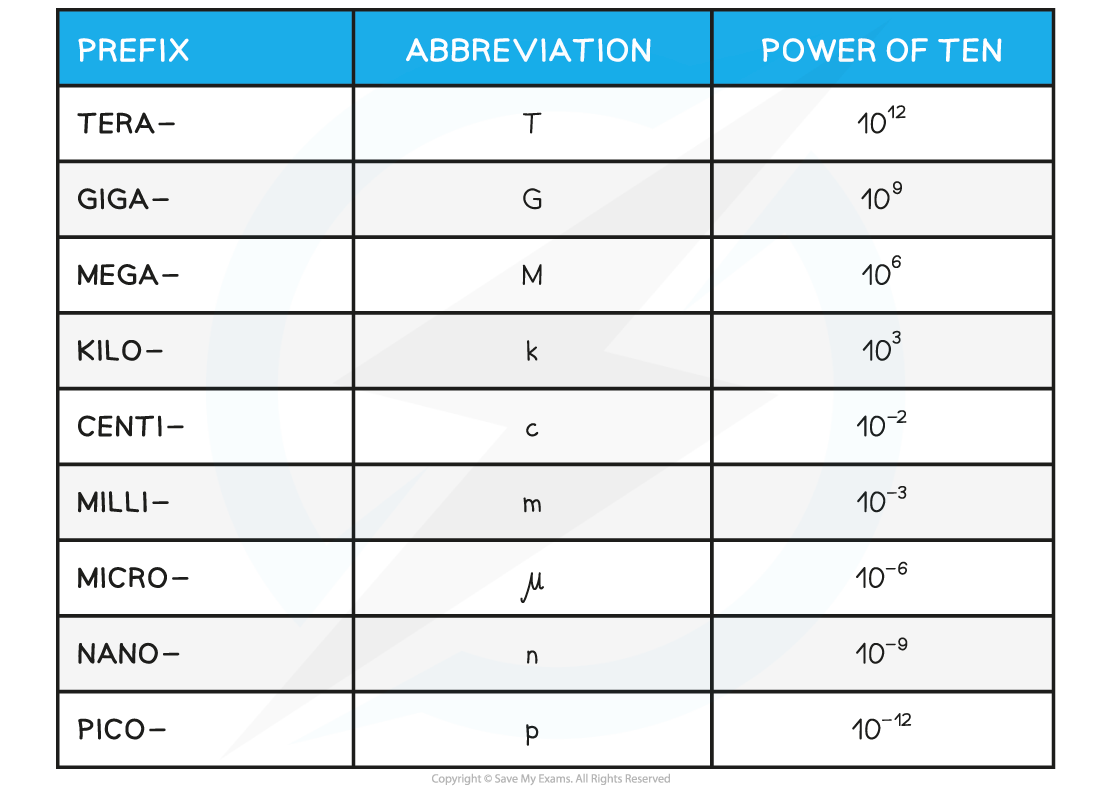
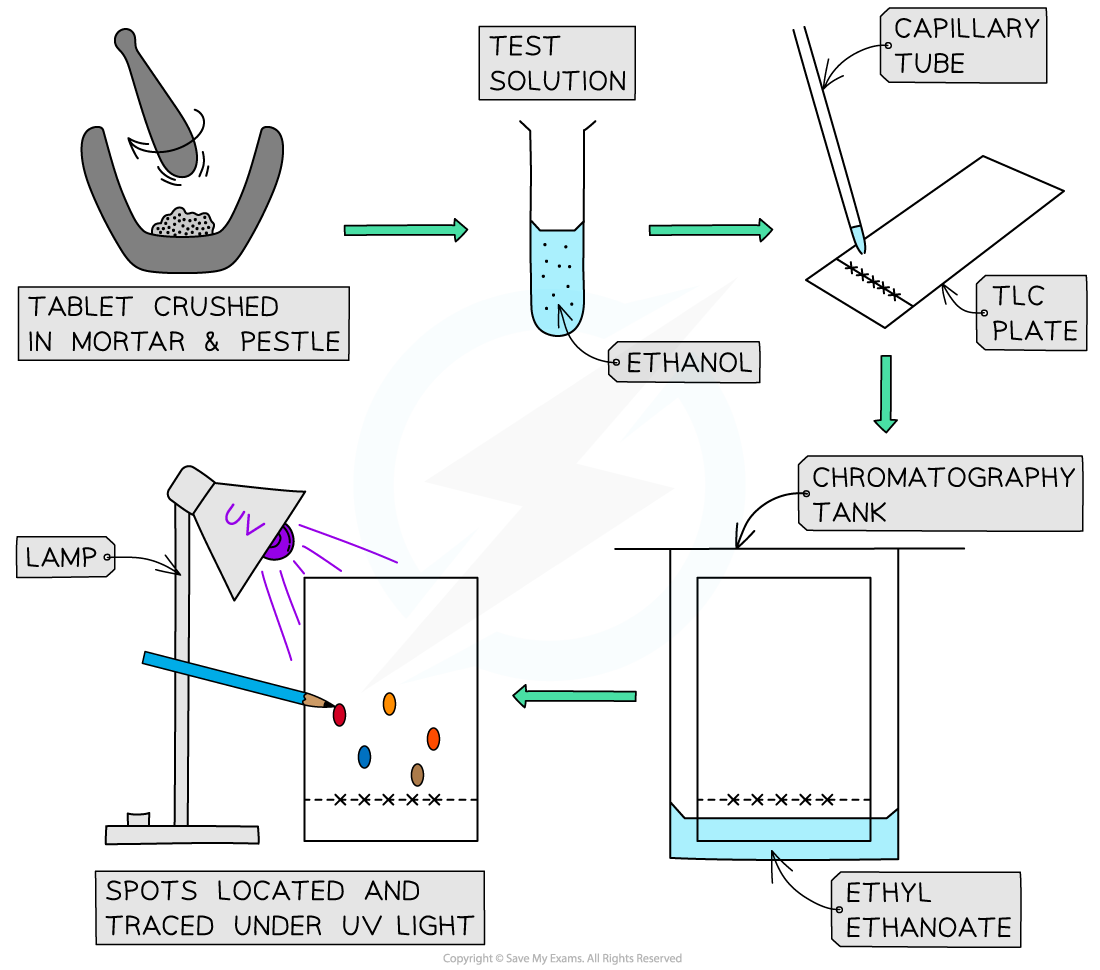
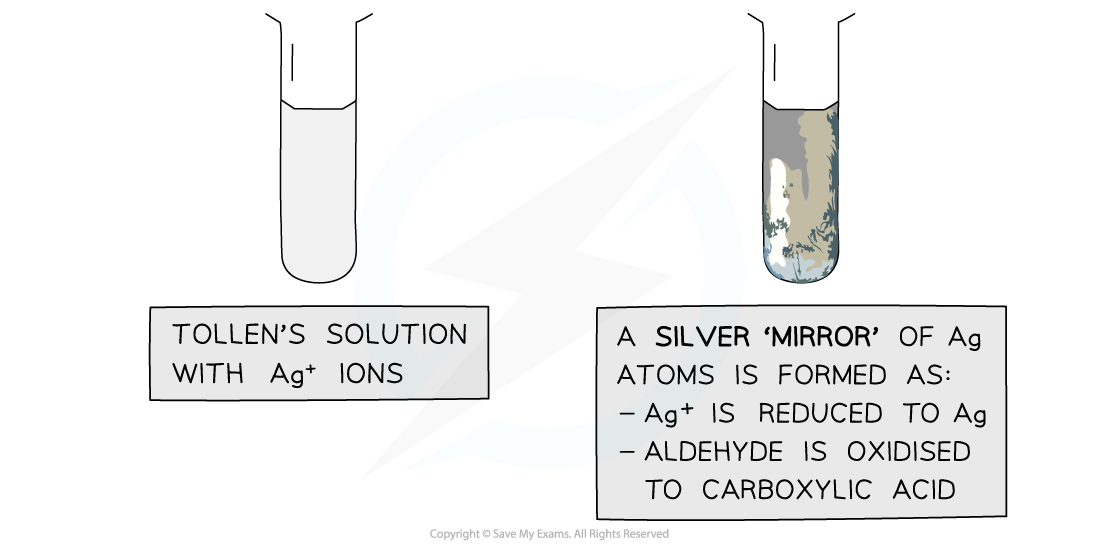
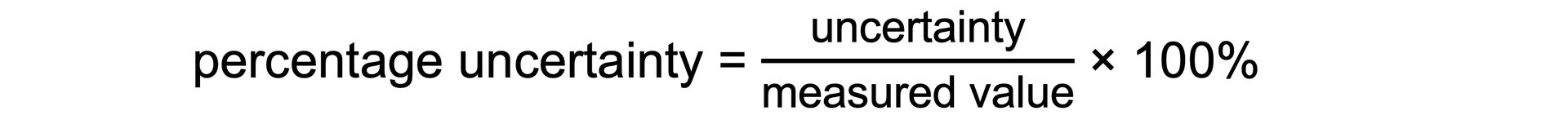AQA A Level Physics复习笔记1.2.3 Determining Uncertainties from Graphs
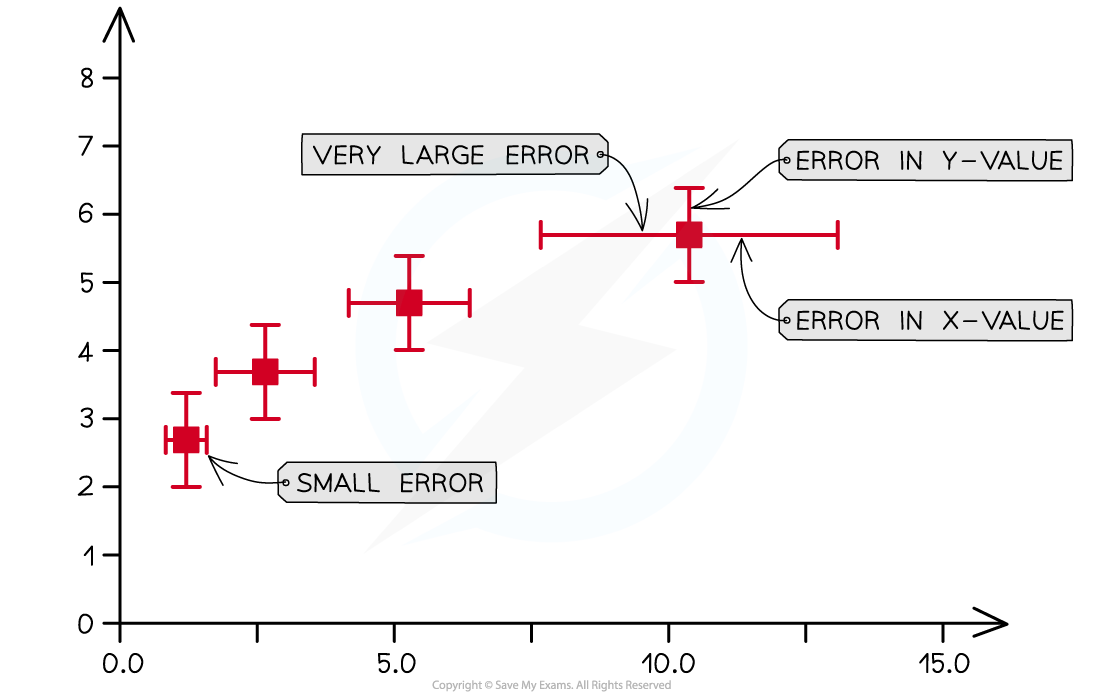
Using Error Bars The uncertainty in a measurement can be shown on a graph as an error bar This bar is drawn above and below the point (or from side to side) and shows the uncertainty in that...