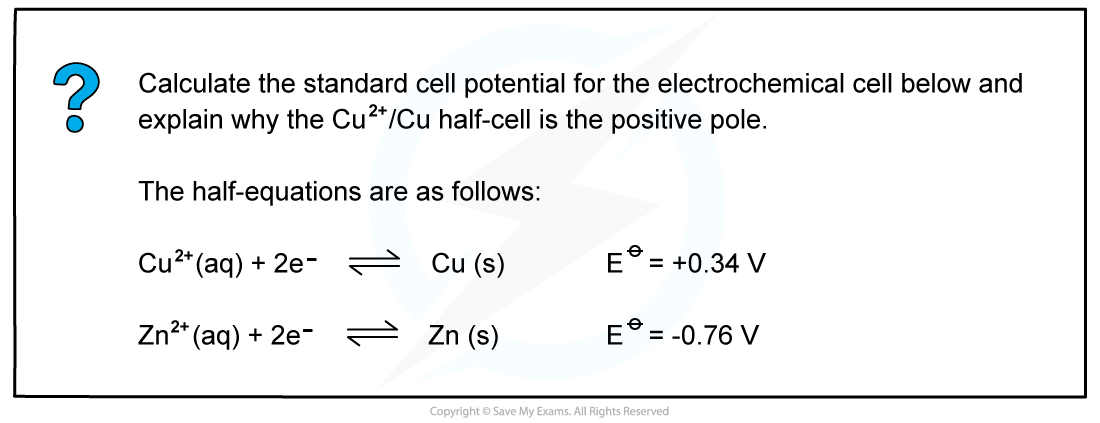
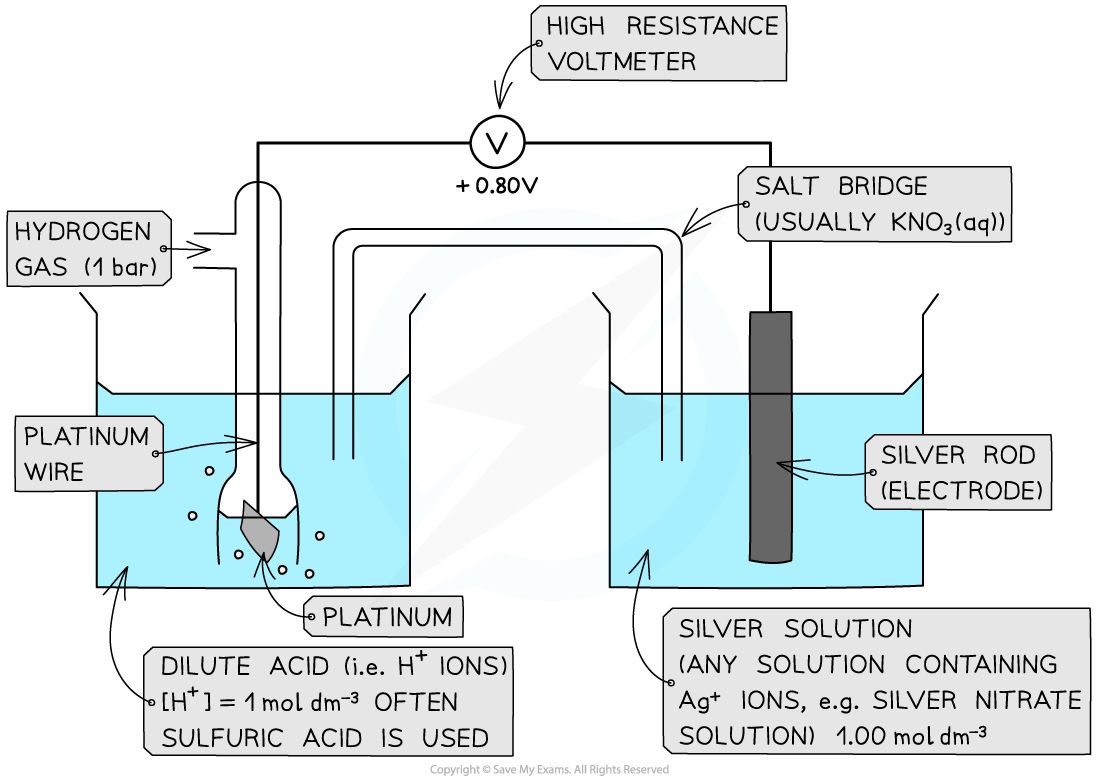
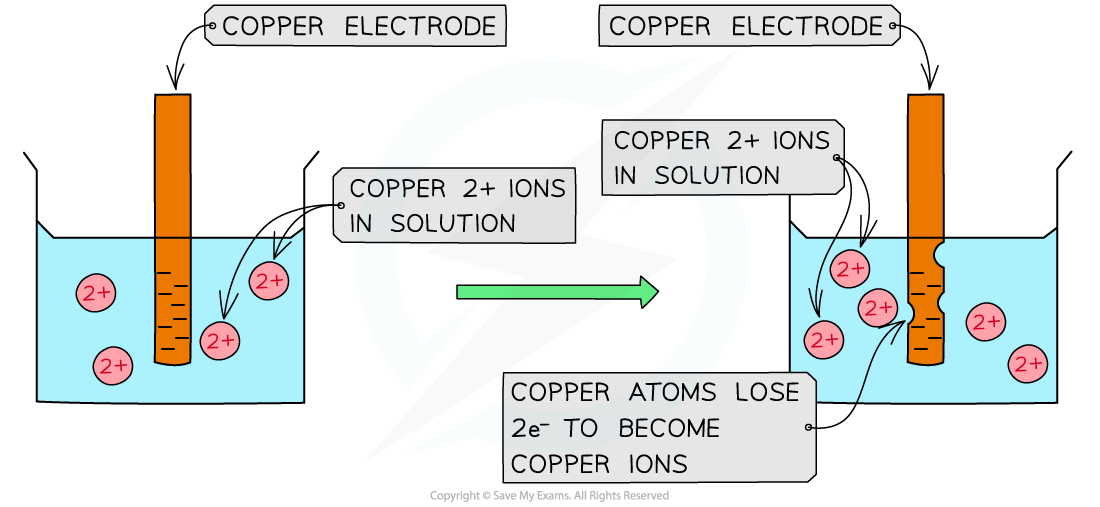
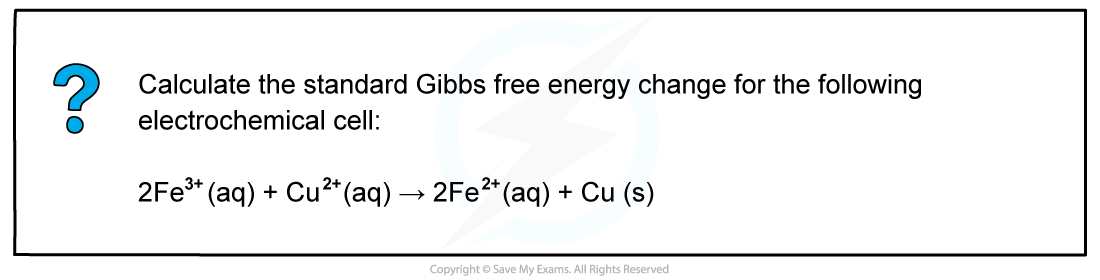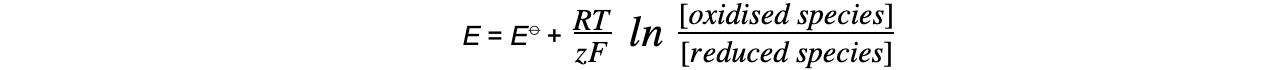CIE A Level Chemistry复习笔记5.4.6 Standard Electrode Potentials: Free Energy Change
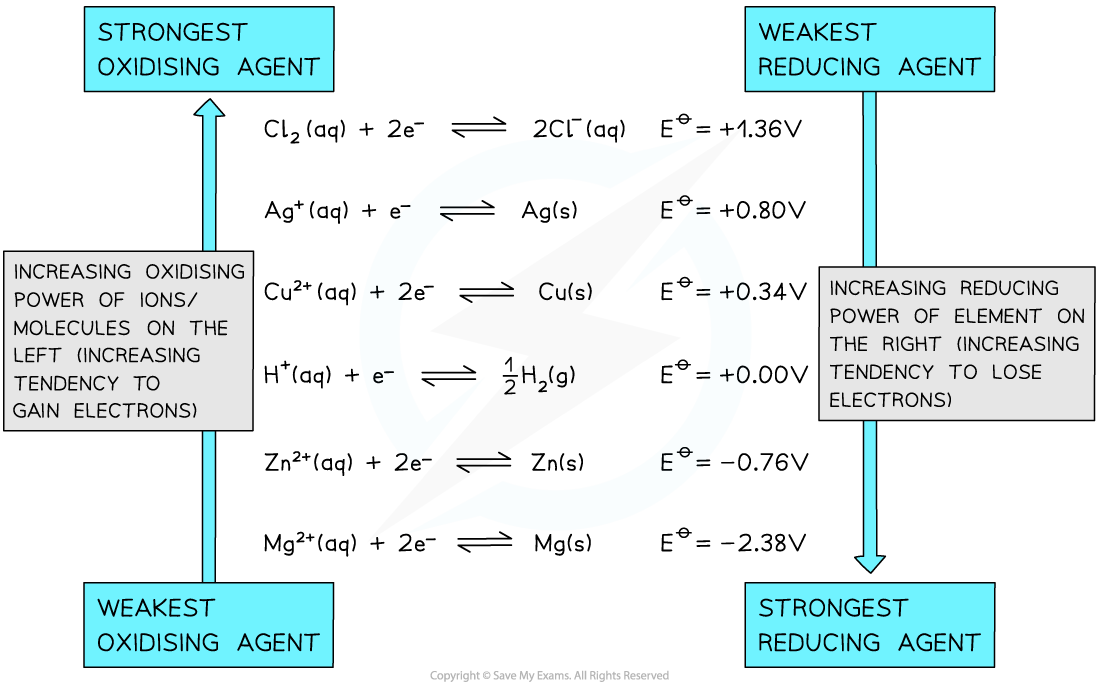
Calculating Free Energy Change Using Standard Electrode Potentials The standard free energy change can be calculated using the standard cell potential of an electrochemical cell ΔGꝋ = - n x Ecellꝋ ...