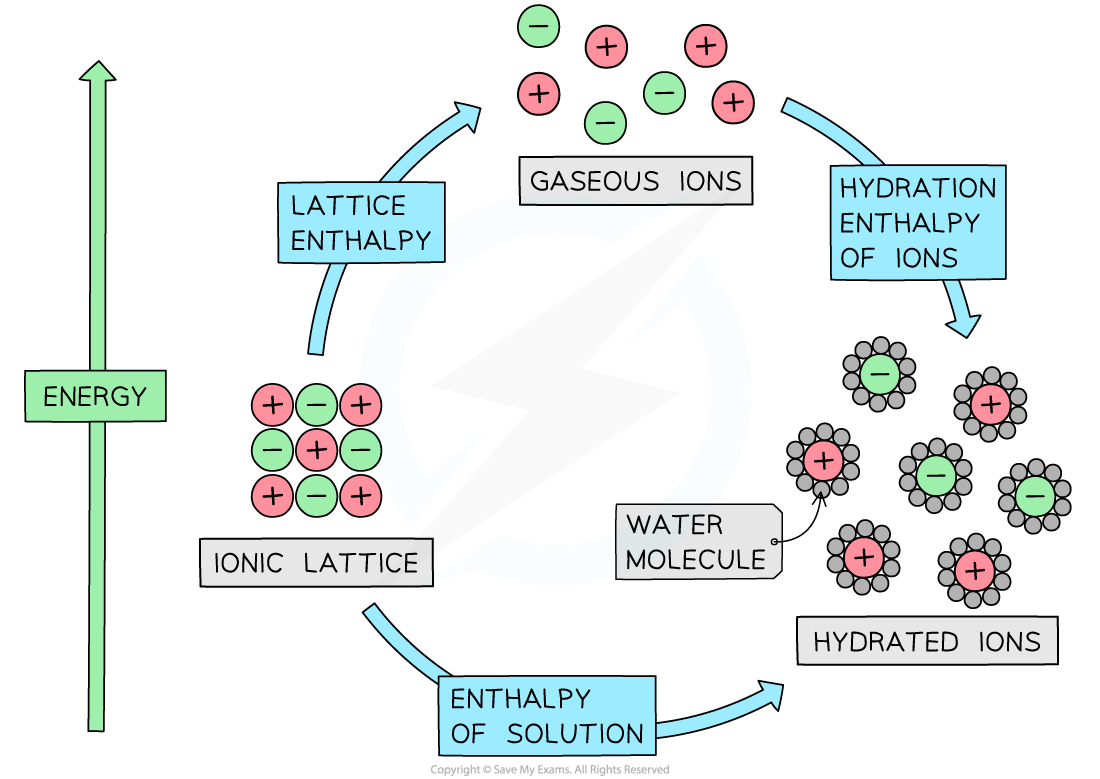
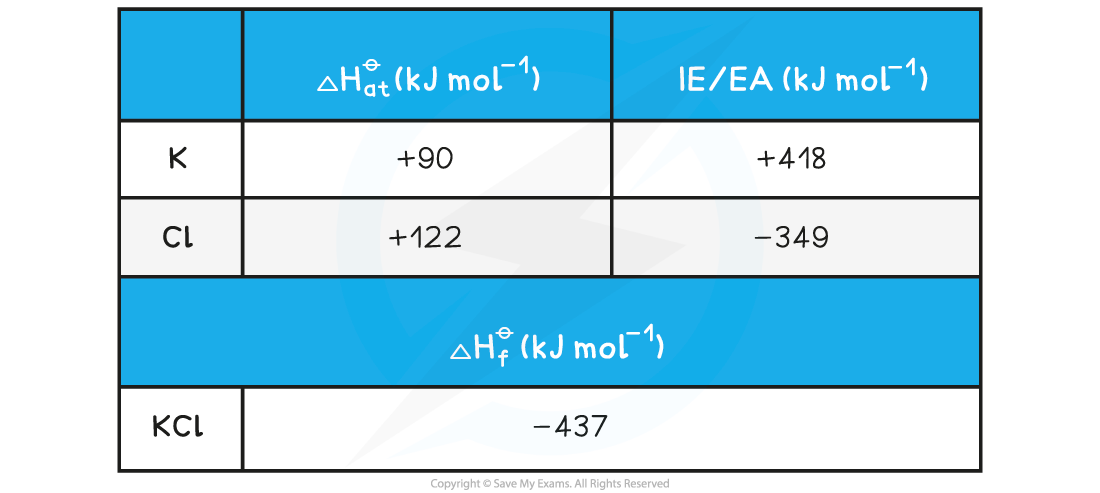
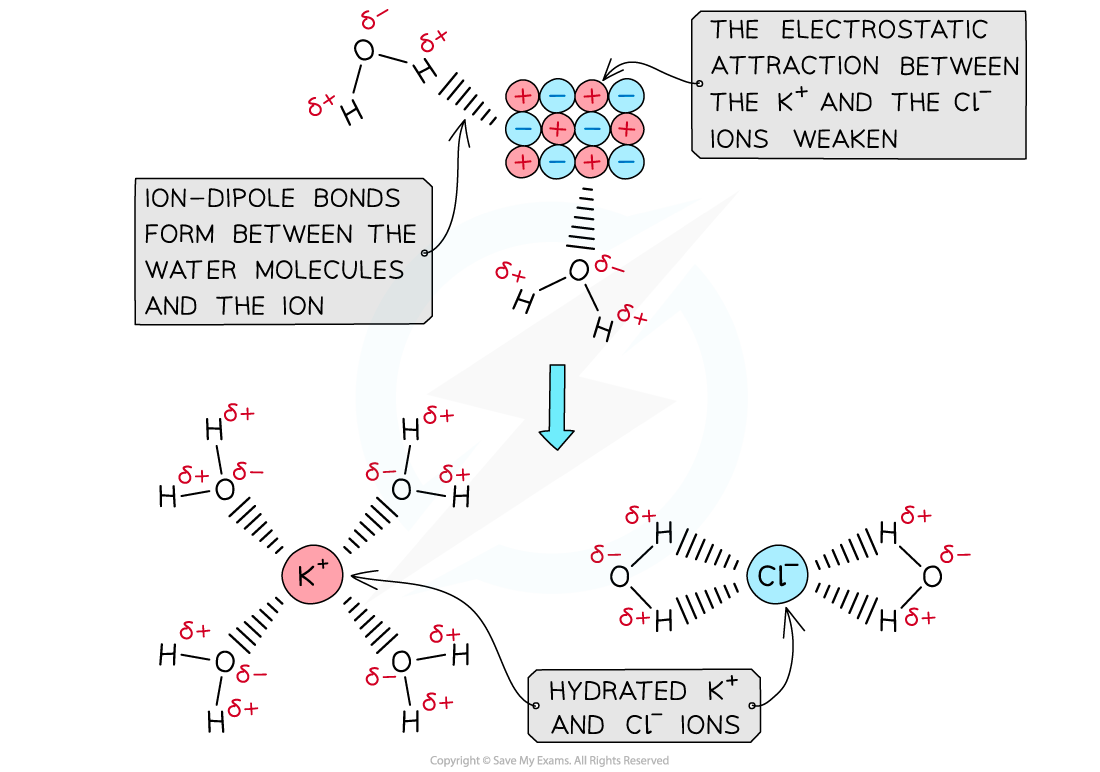
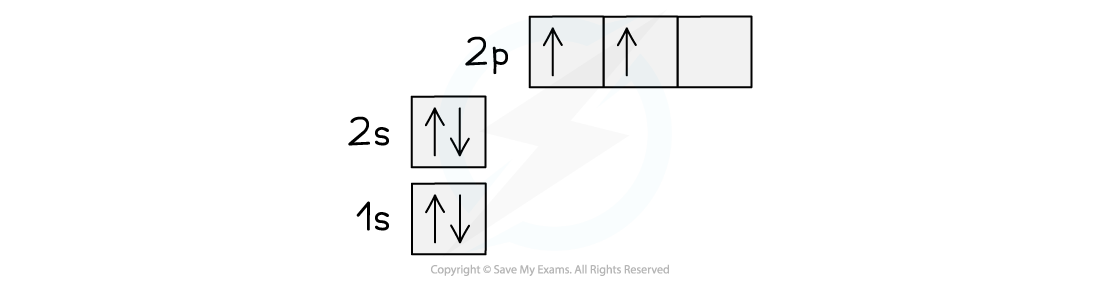
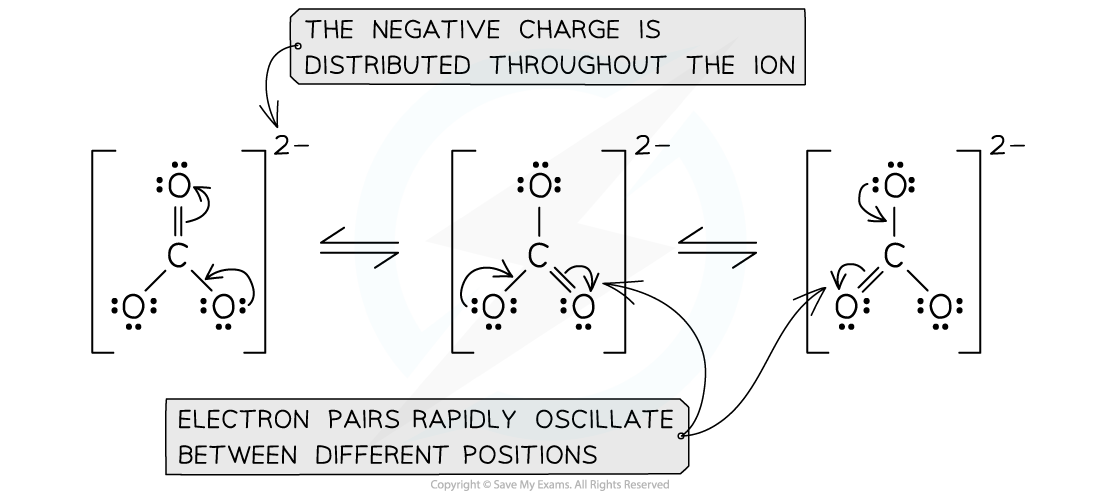
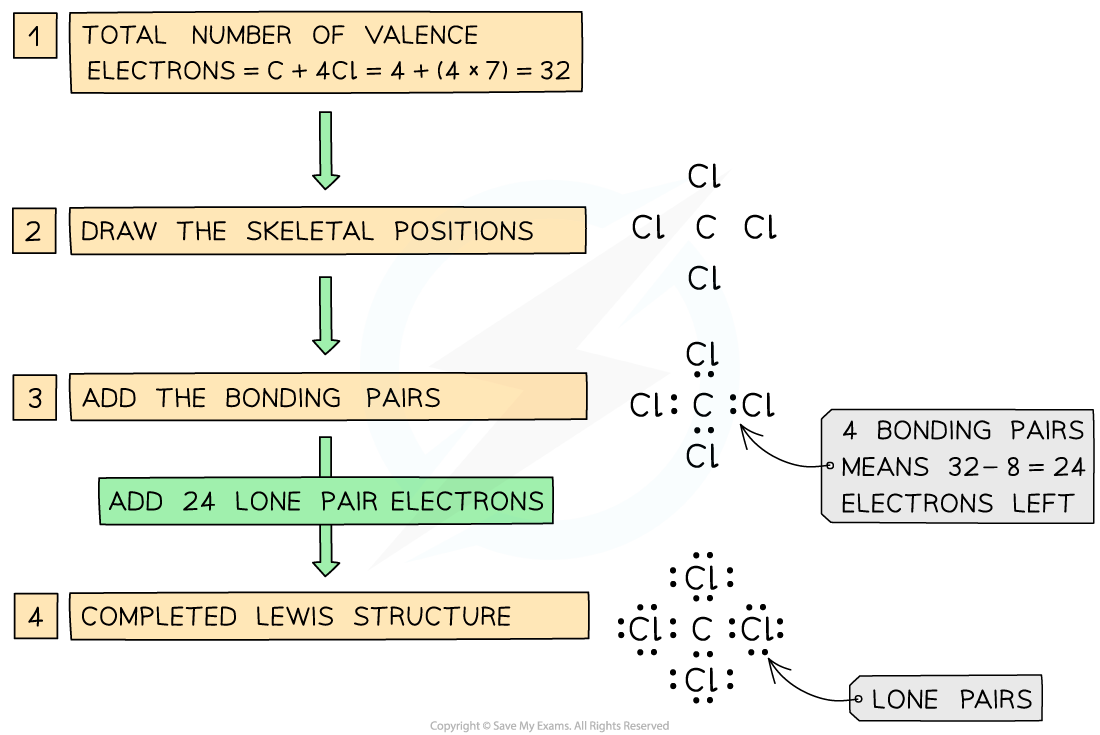
IB DP Chemistry: HL复习笔记15.1.5 Energy Changes in Aqueous Solutions
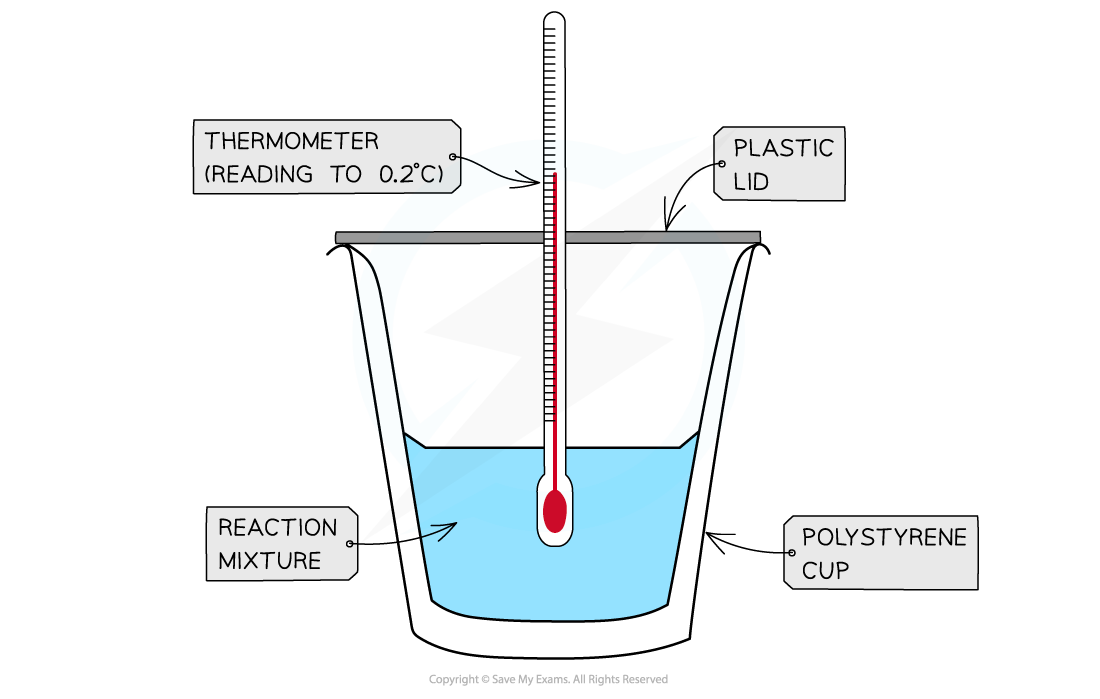
Measuring Energy Changes in the Lab Enthalpies of Solution Calorimetry can be used to find the energy change in chemical reactions. This is much easier to carry out in aqueous solutions We can take...