- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记14.2.1 Delocalisation & Resonance
Delocalisation & Resonance
- The delocalisation of electrons can explain the structures of some species that don’t seem to fit with a Lewis structure
- Delocalised electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or one covalent bond
- The Lewis diagram for the carbonate ion gives a molecule with a double and two single bonds
- There are three possible Lewis structures
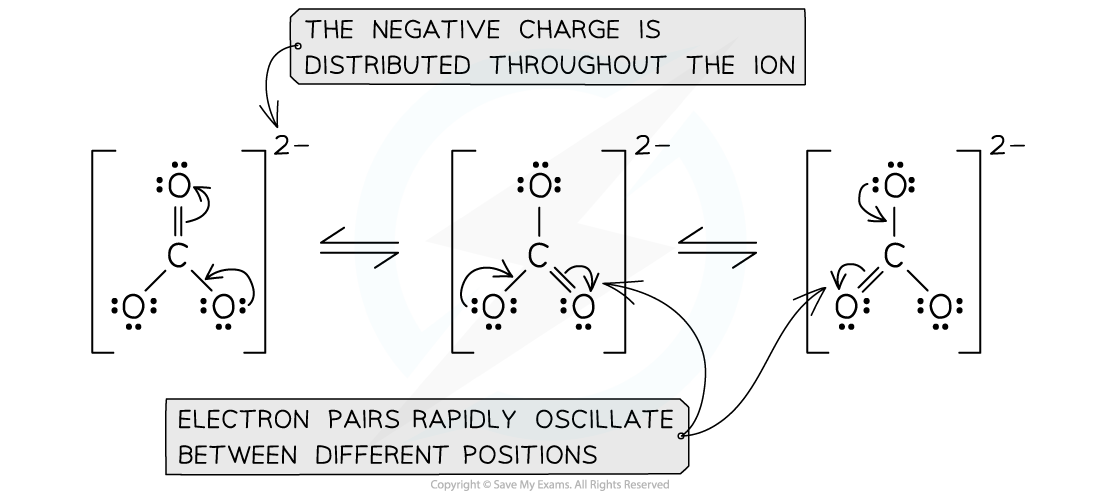
The three resonance structures for the carbonate ion
- These structures are called resonance structures
- However, studies of the electron density and bond length in the carbonate ion indicate all the bonds are equal in length and the electron density is spread evenly between the three oxygen atoms
- The bond length is intermediate between a single and a double bond
- The actual structure is something in between the resonance structures and is known as a resonance hybrid

Resonance hybrid for the carbonate ion
- Dotted lines are used to show the position of the delocalised electrons
- The criteria for forming resonance hybrids structures is that molecules must have a double bond (pi bond) that is capable of migrating from one part of a molecule to another
- This usually arises when there are adjacent atoms with equal electronegativity and lone pairs of electrons that can re-arrange themselves and allow the double bonds to be in different positions
Conjugation & Bond Order
- Structures which have alternative single and double bonds are known as conjugated systems
- Electrons migrate between p-orbitals via adjacent sigma bonds
- The result is a sort of fractional bond, neither a single nor a double, so to accommodate this situation chemists use the concept of bond order:
bond order = total number of bonding pairs ÷ total number of positions
- For example, in the case of the carbonate ion:
bond order in CO32- = total number of CO32- bonding pairs ÷ total number of positions = 4 ÷ 3 = 1.33
- Evidence for bond orders comes from measurements of bond lengths
- A single C-O bond is 143 pm and a double C=O is 122 pm
- The C-O bonds in the carbonate ion are all identical and 129 pm in length which is part way between a single and double
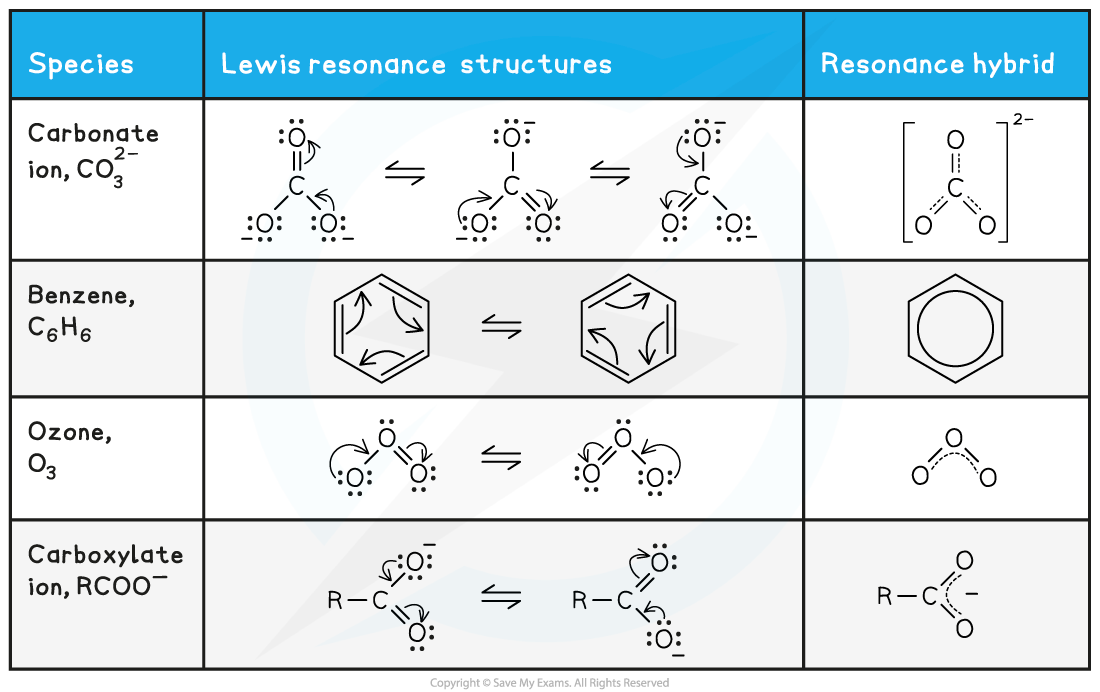
- Other examples that you should know about are benzene, ozone and the carboxylate anion
Resonance hybrids table
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









