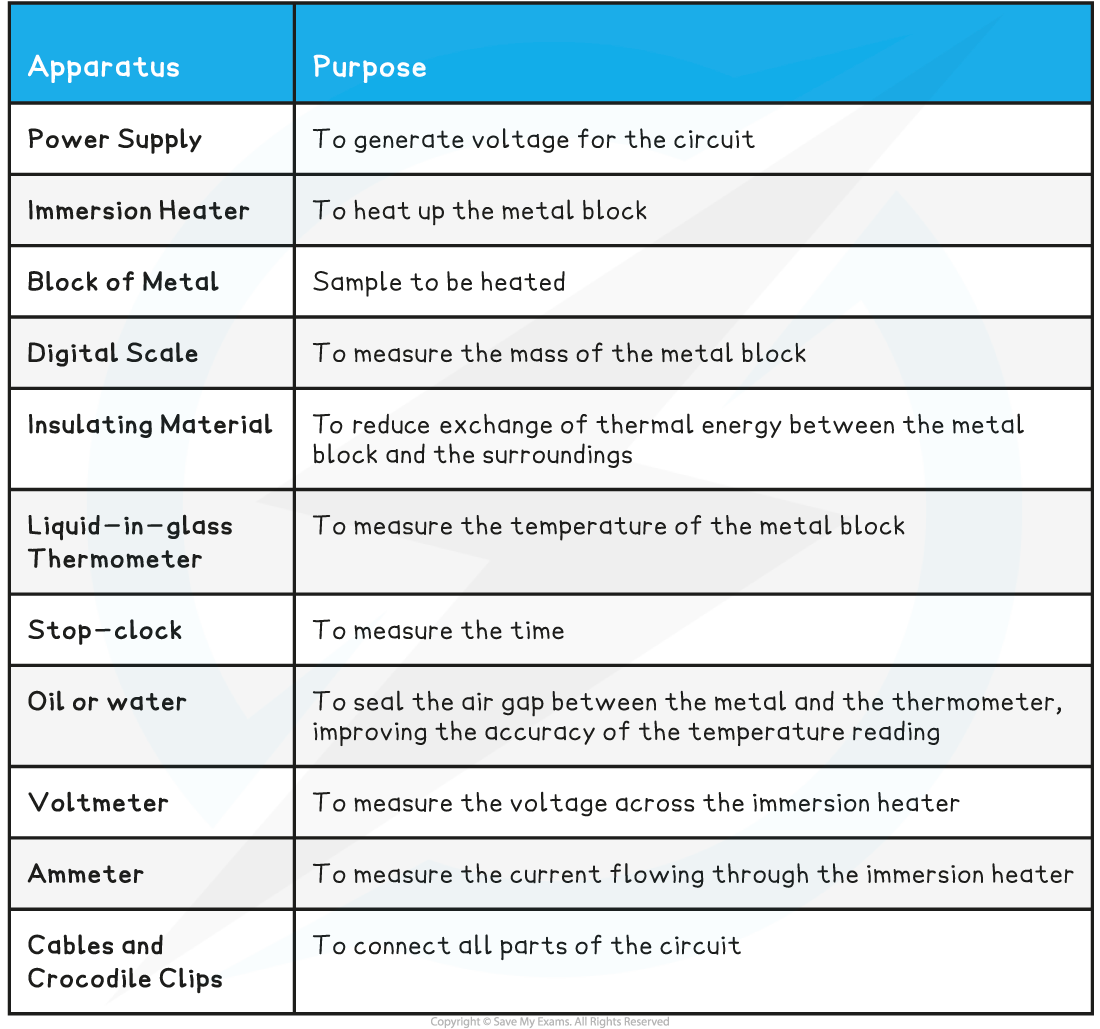
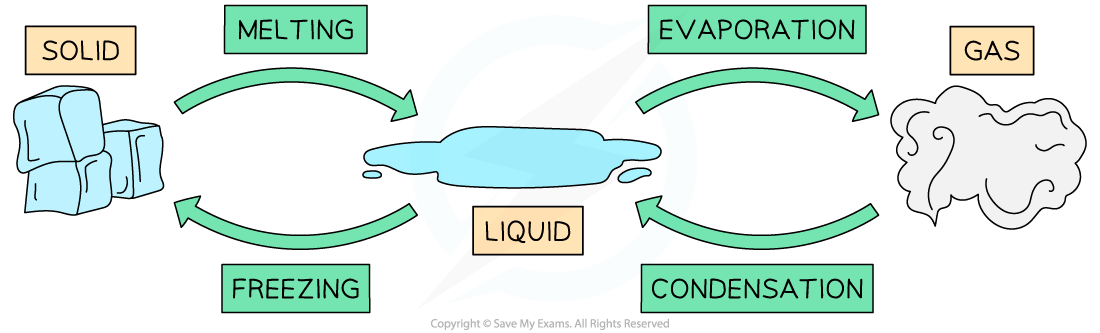
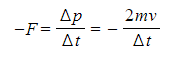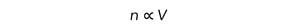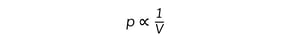IB DP Physics: SL复习笔记4.1.2 Simple Harmonic Oscillations
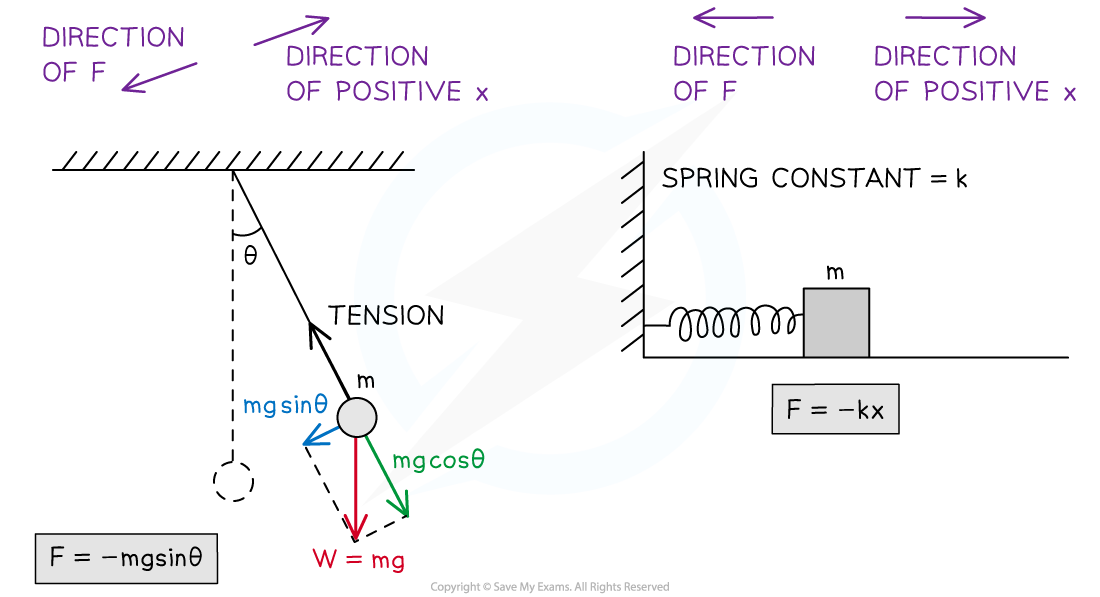
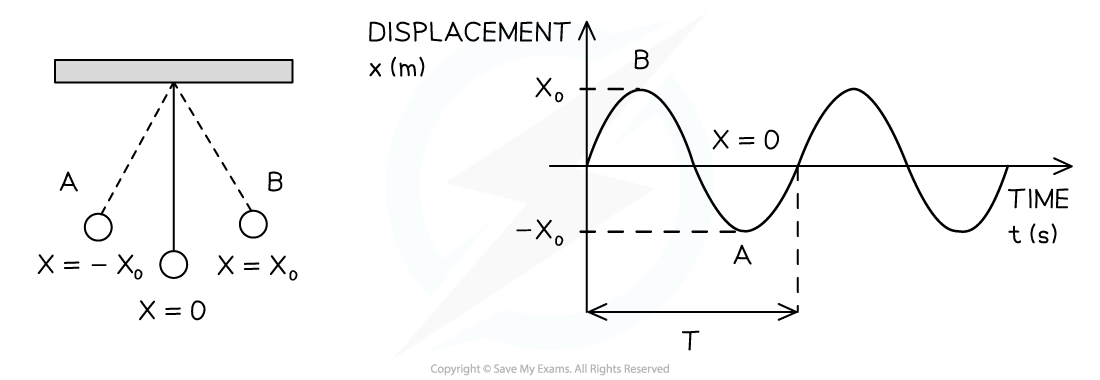
Simple Harmonic Oscillations Simple harmonic motion (SHM) is defined as follows: The motion of an object whose acceleration is directly proportional but opposite in direction to the object's displa...