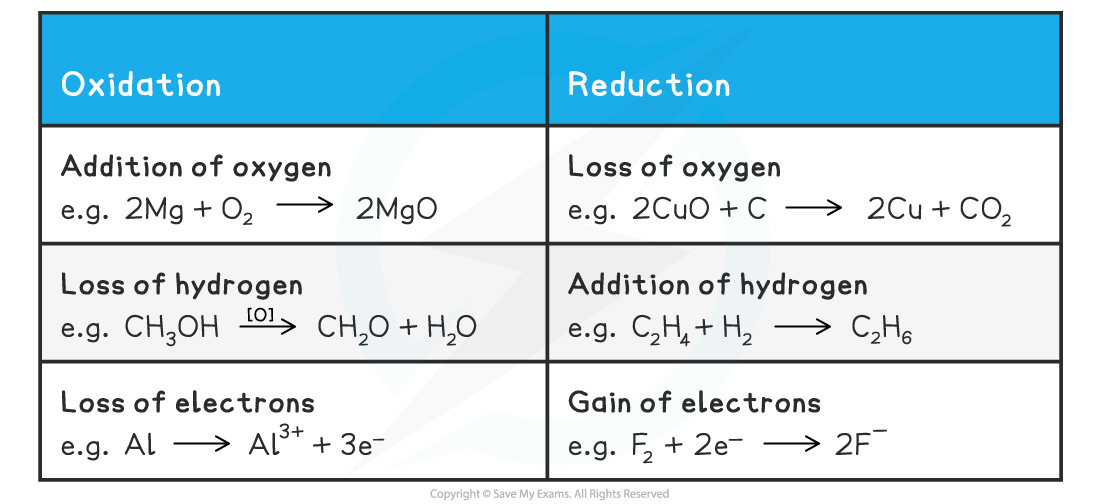
IB DP Chemistry: HL复习笔记9.1.2 Deducing Oxidation Numbers
Deducing Oxidation Numbers The oxidation numbers of all other atoms in their compounds can vary By following the oxidation number rules, the oxidation number of any atom in a compound or ion can be...