Edexcel A Level Chemistry:复习笔记6.1.3 Measuring Standard Electrode Potential
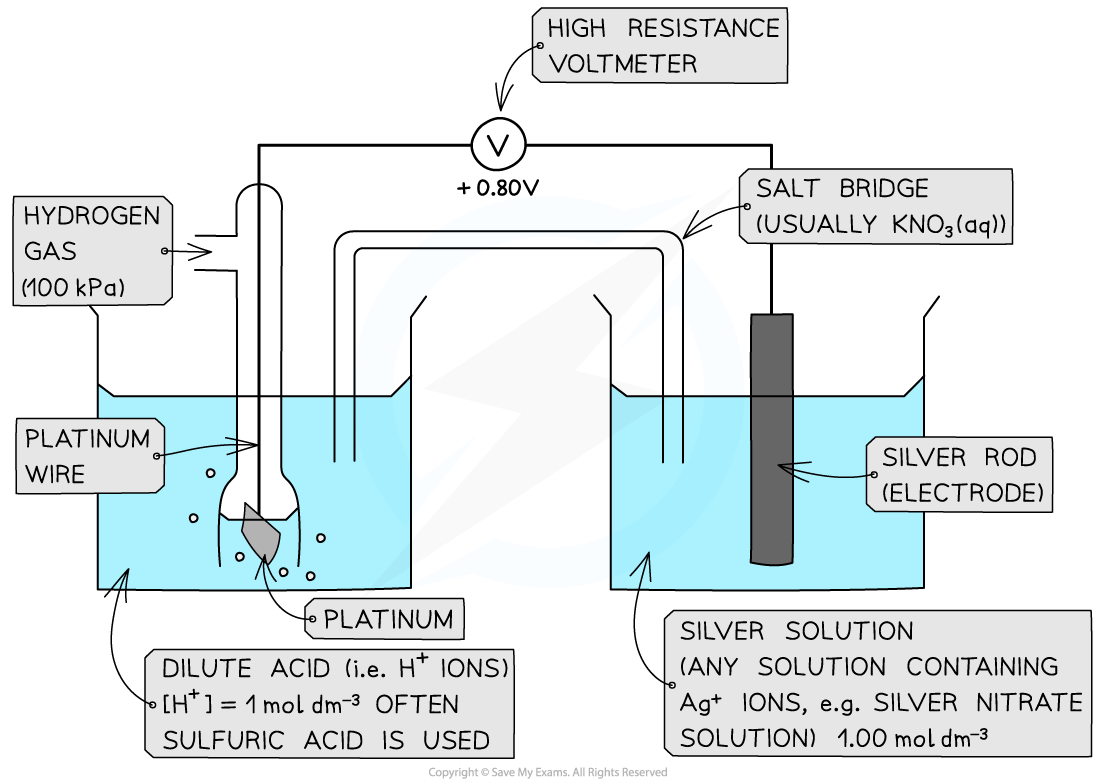
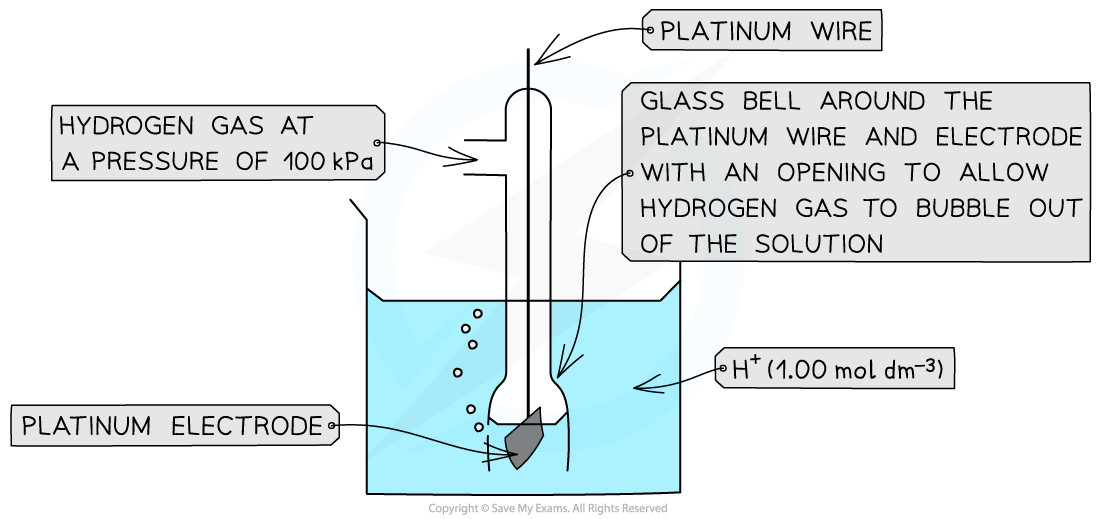
Measuring Standard Electrode Potential There are three different types of half-cells that can be connected to a standard hydrogen electrode to measure standard electrode potential A metal / ...