- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记3.1.4 Structural Isomerism
Structural Isomerism
- Structural isomers are compounds that have the same molecular formula but different structural formulae
- E.g. propene and cyclopropane
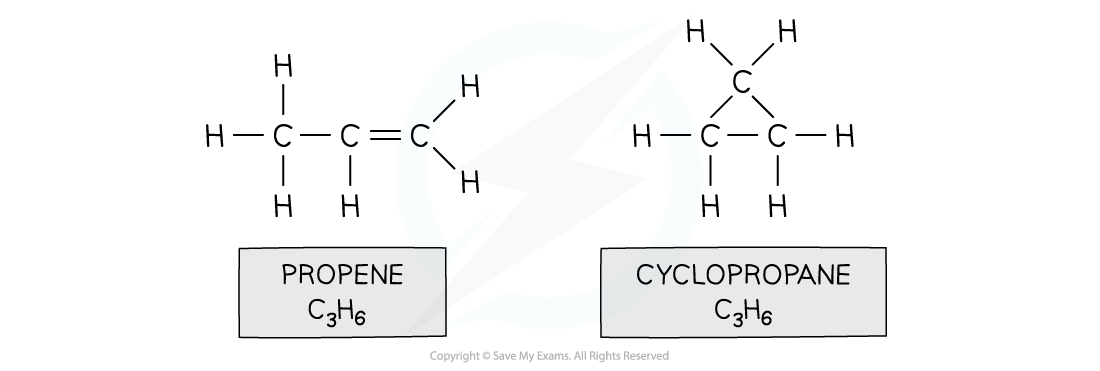
Both propene and cyclopropane are made up of 3 carbon and 6 hydrogen atoms but the structure of the two molecules differs
- There are two different types of structural isomerism you need to be aware of:
- Chain isomerism
- Positional isomerism
Chain isomerism
- Chain isomerism is when compounds have the same molecular formula, but their longest hydrocarbon chain is not the same
- This is caused by branching
- E.g. pentane and 2,2-dimethylpropane
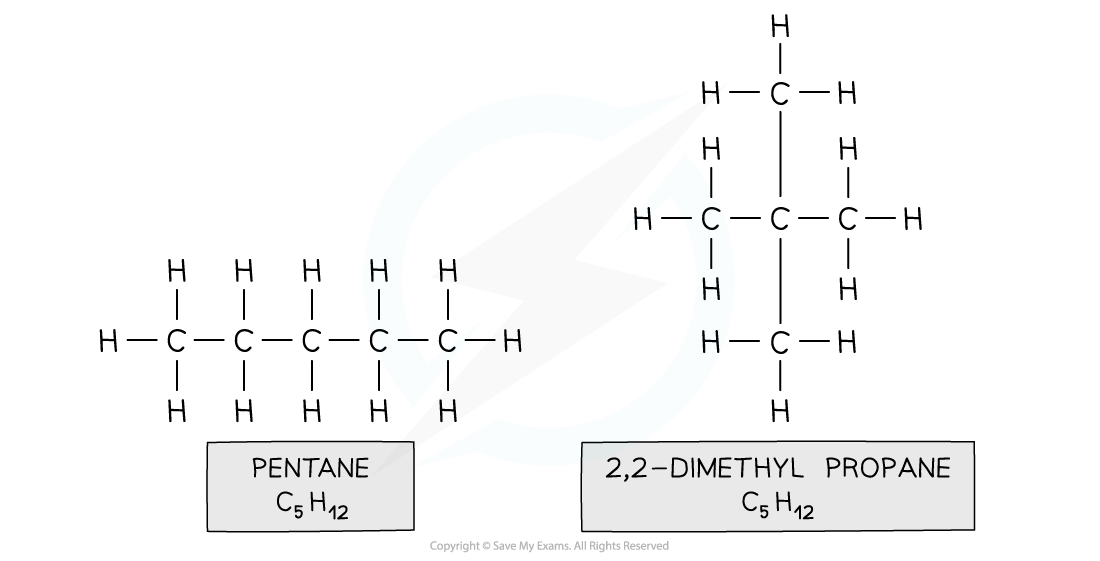
Both compounds are made up of the same atoms, however the longest carbon chain in pentane is 5 and in 2,2-dimethylpropane it is 3 (with two methyl branches)
Positional isomerism
- Positional isomers arise from differences in the position of a functional group in each isomer
- The functional group can be located on different carbons
- For example, butan-1-ol and butan-2-ol
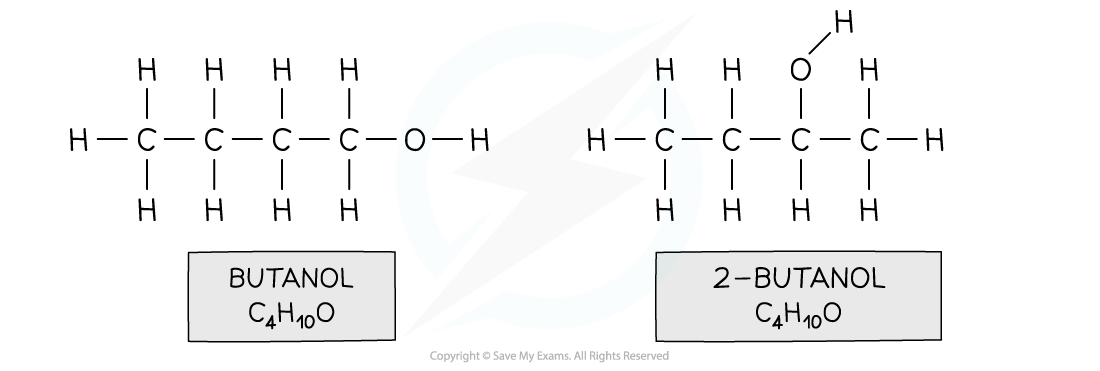
Both compounds have an alcohol group and are made up of 4 carbons, 10 hydrogens and one oxygen, however in butanol the functional group is located on the first carbon and in 2-butanol on the second carbon
Worked Example
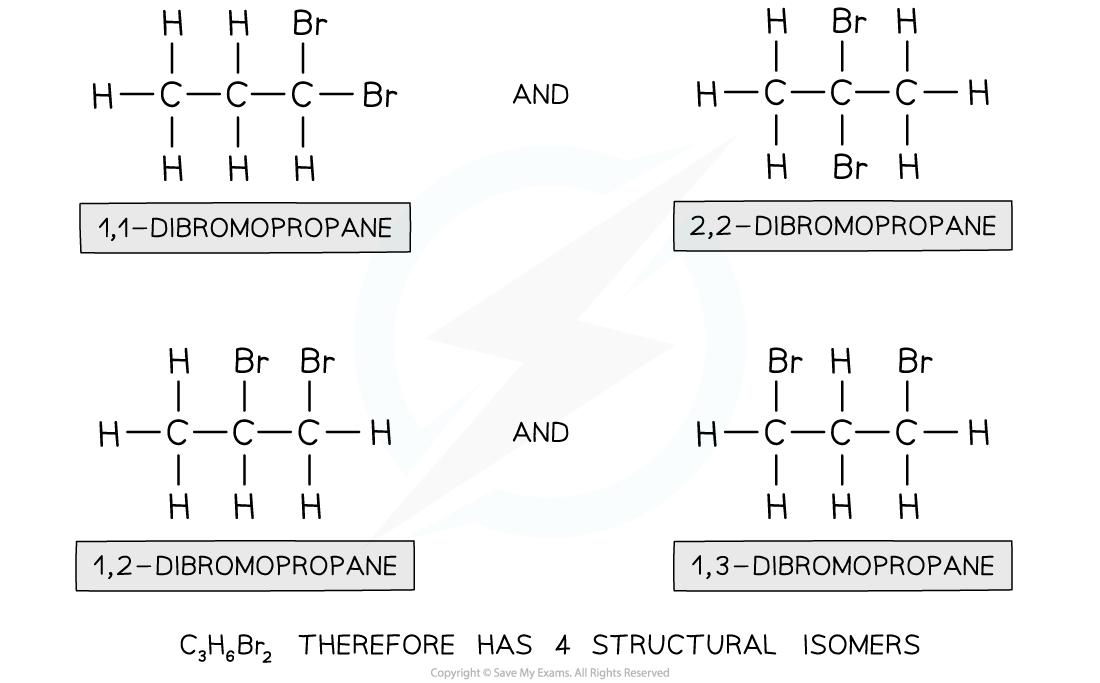
Isomers of dibromopropane
How many isomers are there of dibromopropane, C3H6Br2?
Answer
Step 1: Draw the structural formula of the compound

Step 2: Determine whether it is a stereo or structural isomer
There is no restricted bond rotation around the C-C bond, so it is structural isomerism
Step 3: Determine whether it is a functional group, chain or positional isomerism
-
-
- Functional group? No, as Br is the only functional group possible
- Chain? No, as the longest chain can only be 3
- Positional? Yes, as the two bromine atoms can be bonded to different carbon atoms
-

Worked Example
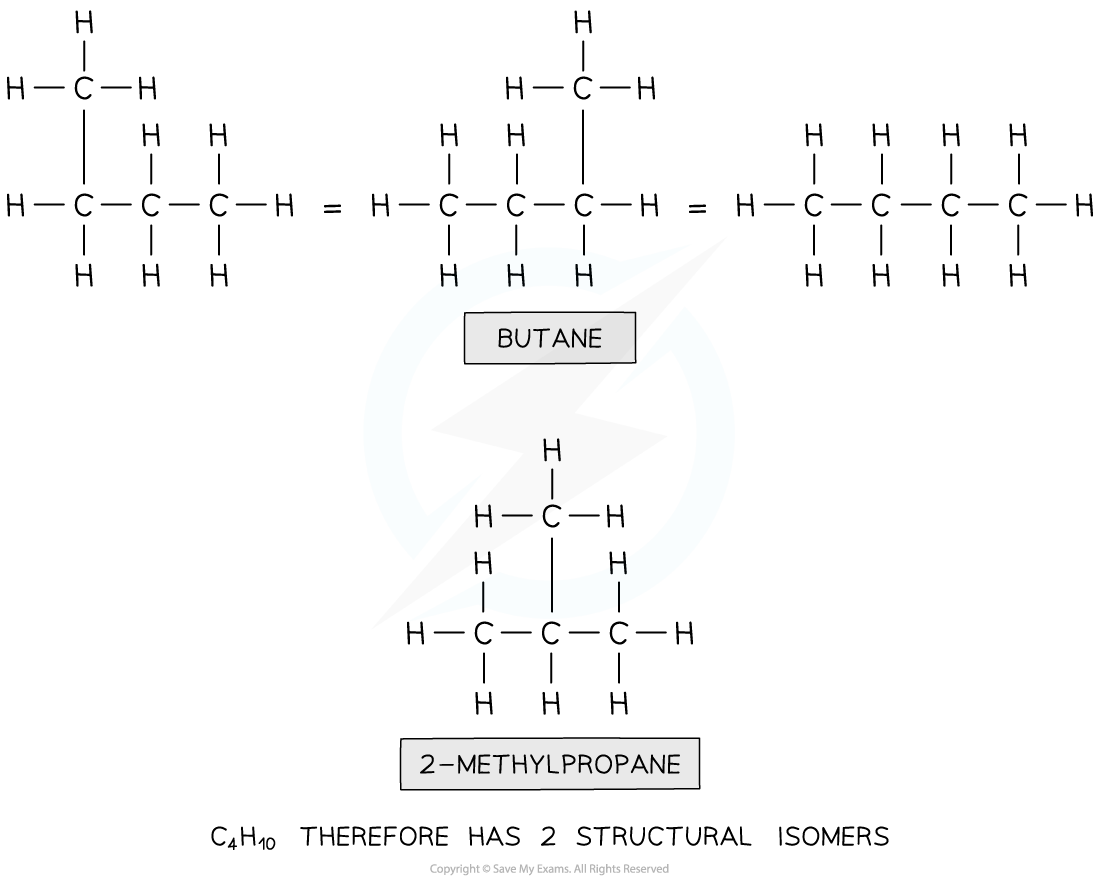
Deducing isomers of C4H10
How many isomers are there of the compound with molecular formula C4H10?
Answer
Step 1: Draw the structural formula of the compound

Step 2: Determine whether it is a stereo or structural isomer.
There is no restricted bond rotation around the C-C bond so it is structural isomerism
Step 3: Determine whether it is a functional group, chain or positional isomerism
-
-
- Functional group? No, as there are no functional groups
- Positional? No, as there are no functional groups which can be positioned on different carbon atoms
- Chain? Yes!
-
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









