- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.5.1 Electronegativity
Defining Electronegativity
- Electronegativity is the power of an atom to attract the pair of electrons in a covalent bond towards itself
- The electron distribution in a covalent bond between elements with different electronegativities will be unsymmetrical
- This phenomenon arises from the positive nucleus’s ability to attract the negatively charged electrons, in the outer shells, towards itself
- The Pauling scale is used to assign a value of electronegativity for each atom
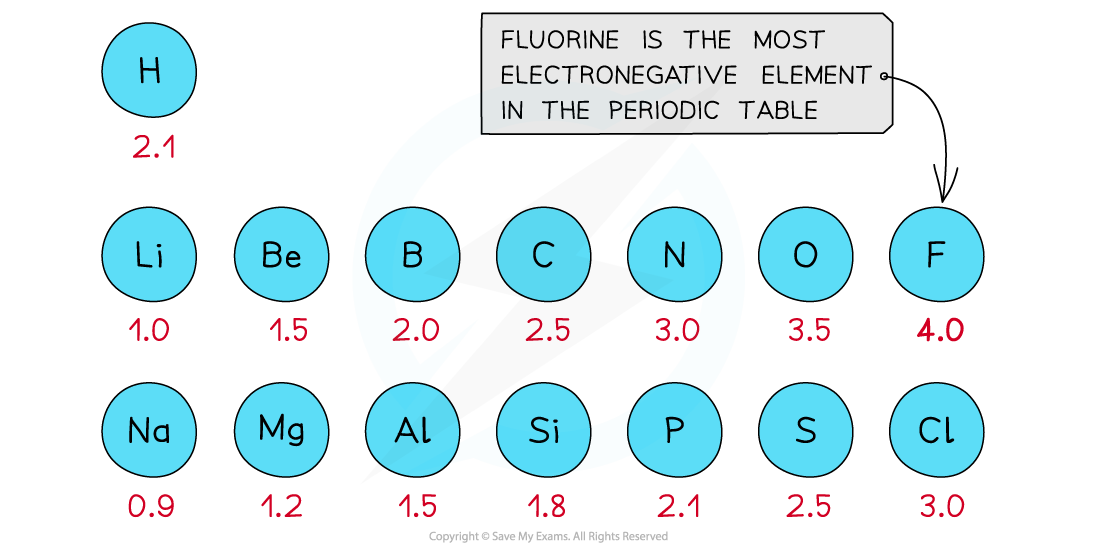
First three rows of the periodic table showing electronegativity values
- Fluorine is the most electronegative atom on the Periodic Table, with a value of 4.0 on the Pauling Scale
- It is best at attracting electron density towards itself when covalently bonded to another atom
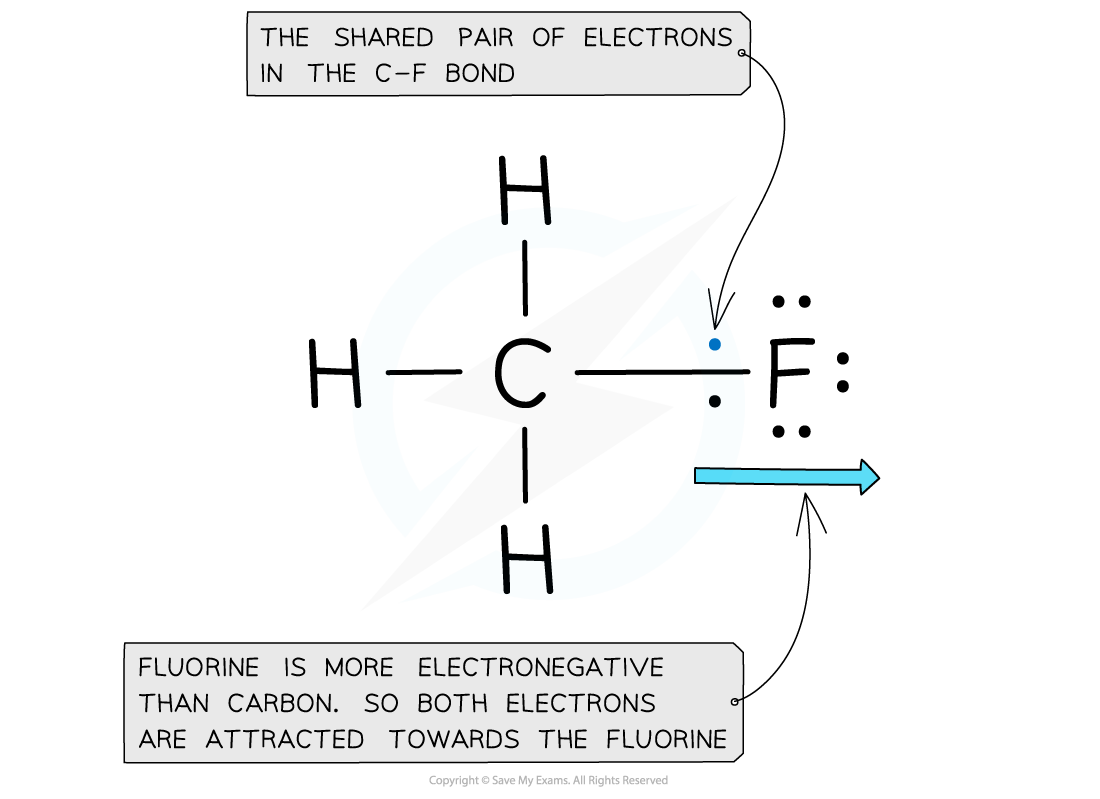
Electron distribution in the C-F bond of fluoromethane
Nuclear charge
- Attraction exists between the positively charged protons in the nucleus and negatively charged electrons found in the energy levels of an atom
- An increase in the number of protons leads to an increase in nuclear attraction for the electrons in the outer shells
- Therefore, an increased nuclear charge results in an increased electronegativity
Atomic radius
- The atomic radius is the distance between the nucleus and electrons in the outermost shell
- Electrons closer to the nucleus are more strongly attracted towards its positive nucleus
- Those electrons further away from the nucleus are less strongly attracted towards the nucleus
- Therefore, an increased atomic radius results in a decreased electronegativity
Shielding
- Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus
- Therefore, the addition of extra shells and subshells in an atom will cause the outer electrons to experience less of the attractive force of the nucleus
- Sodium (period 3, group 1) has higher electronegativity than caesium (period 6, group 1) as it has fewer shells and therefore the outer electrons experience less shielding than in caesium
- Thus, an increased number of inner shells and subshells will result in a decreased electronegativity
Trends in electronegativity
- Electronegativity varies across periods and down the groups of the periodic table
Down a group
- There is a decrease in electronegativity going down the group
- The nuclear charge increases as more protons are being added to the nucleus
- However, each element has an extra filled electron shell, which increases shielding
- The addition of the extra shells increases the distance between the nucleus and the outer electrons resulting in larger atomic radii
- Overall, there is decrease in attraction between the nucleus and outer bonding electrons
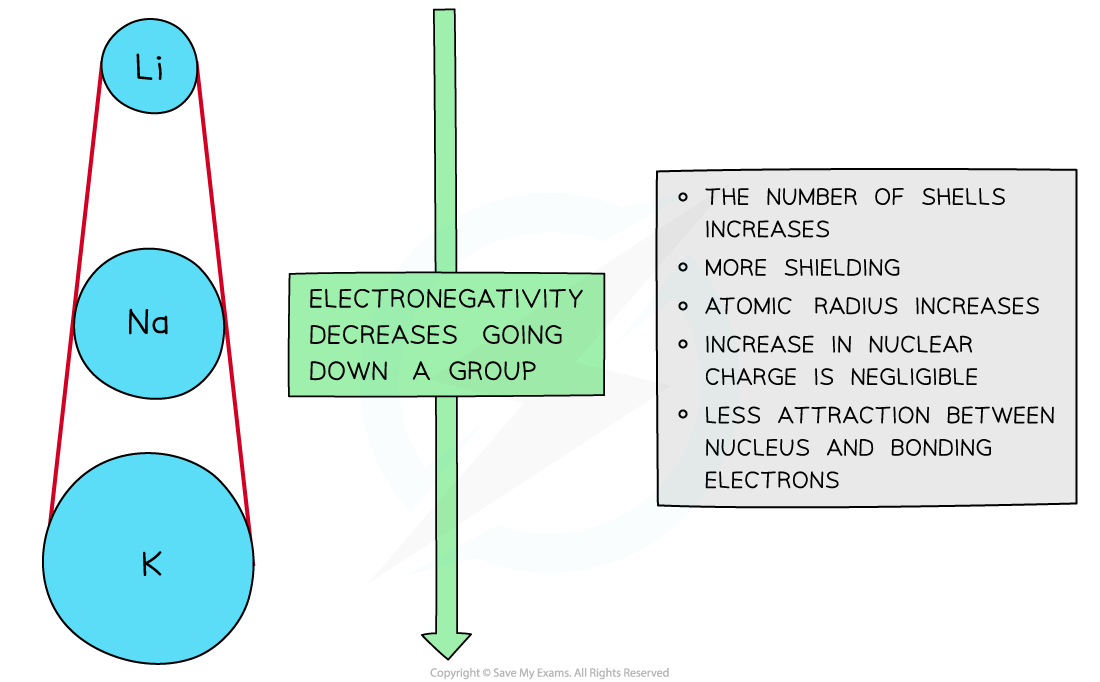
Electronegativity decreases going down the groups of the periodic table
Across a period
- Electronegativity increases across a period
- The nuclear charge increases with the addition of protons to the nucleus
- Shielding remains relatively constant across the period as no new shells are being added to the atoms
- The nucleus has an increasingly strong attraction for the bonding pair of electrons of atoms across the period of the periodic table
- This results in smaller atomic radii
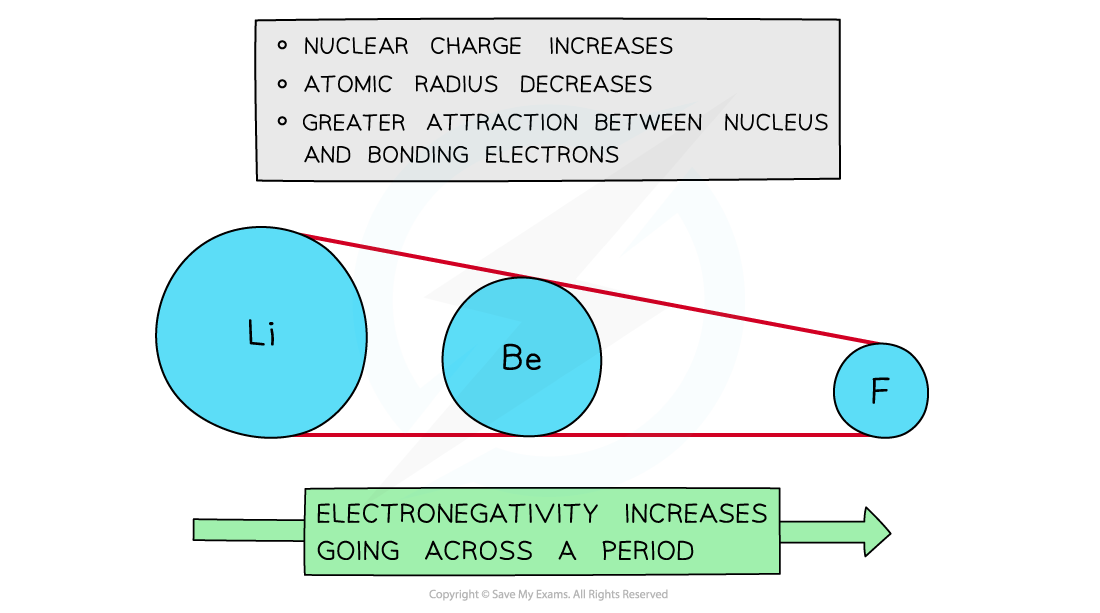
Electronegativity increases going across the periods of the Periodic Table
Bond Polarity
- When two atoms in a covalent bond have the same electronegativity the covalent bond is nonpolar
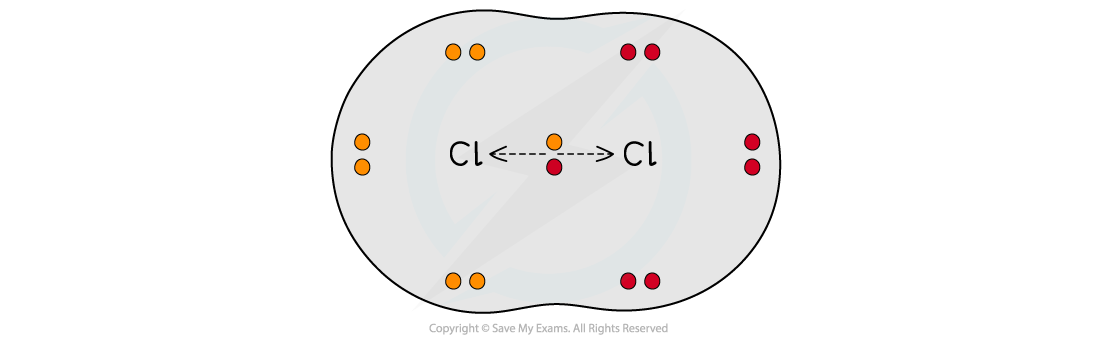
The two chlorine atoms have the same electronegativities so the bonding electrons are shared equally between the two atoms
- The difference in electronegativities will dictate the type of bond that is formed
- When the electronegativities are very different (difference of more than 1.7) then ions will be formed and the bond will be ionic
- When two atoms in a covalent bond have a difference in electronegativities of 0.3 to 1.7 a covalent bond is formed and the bond will be polar
- The electrons will be drawn towards the more electronegative atom
- As a result of this:
- The negative charge centre and positive charge centre do not coincide with each other
- This means that the electron distribution is asymmetric
- The less electronegative atom gets a partial charge of δ+ (delta positive)
- The more electronegative atom gets a partial charge of δ- (delta negative)
- The greater the difference in electronegativity the more polar the bond becomes
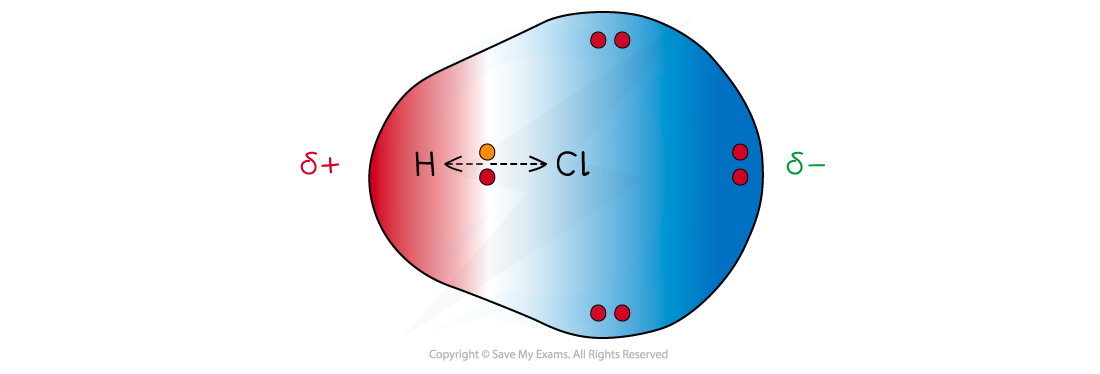
Cl has a greater electronegativity than H causing the electrons to be more attracted towards the Cl atom which becomes delta negative and the H delta positive
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









