- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.1.7 Constructing Energy Cycles using Enthalpy Changes & Lattice Energy
Energy Cycle Using Enthalpy Changes & Lattice Energy
- The standard enthalpy change of hydration (ΔHhydꝋ) can be calculated by constructing energy cycles and applying Hess’s law
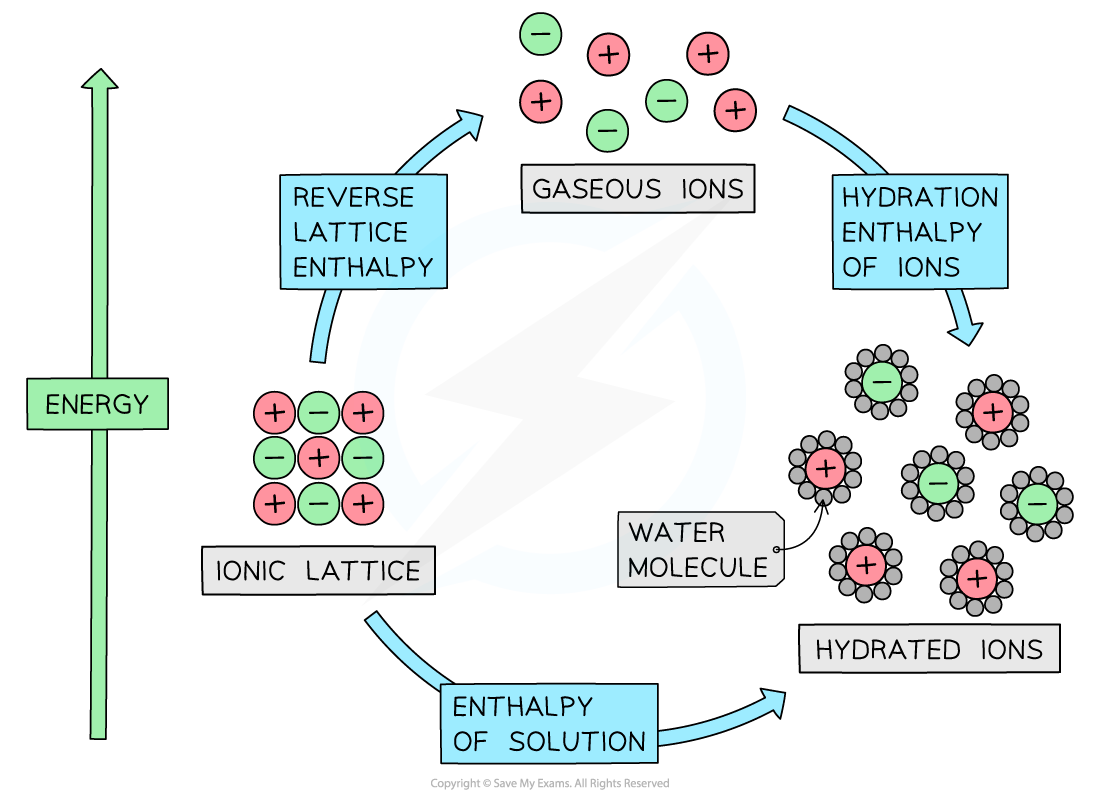
Energy cycle involving enthalpy change of solution, lattice energy, and enthalpy change of hydration
- The energy cycle shows that there are two routes to go from the ionic lattice to the hydrated ions in an aqueous solution:
- Route 1: going from ionic solid → ions in aqueous solution (this is the direct route)
ΔHsolꝋ= Enthalpy of solution
- Route 2: going from ionic lattice → gaseous ions → ions in aqueous solution (this is the indirect route)
-ΔHlattꝋ + ΔHhydꝋ = reverse lattice enthalpy + hydration enthalpies
Lattice enthalpy usually means Lattice formation enthalpy, in other words bond forming. If we are breaking the lattice then this is reversing the enthalpy change so a negative sign is added in front of the term (alternatively it is called lattice dissociation enthalpy)
- According to Hess’s law, the enthalpy change for both routes is the same, such that:
ΔHsolꝋ = -ΔHlattꝋ + ΔHhydꝋ
ΔHhydꝋ = ΔHsolꝋ + ΔHlattꝋ
- Each ion will have its own enthalpy change of hydration, ΔHhydꝋ, which will need to be taken into account during calculations
- The total ΔHhydꝋ is found by adding the ΔHhydꝋ values of both anions and cations together
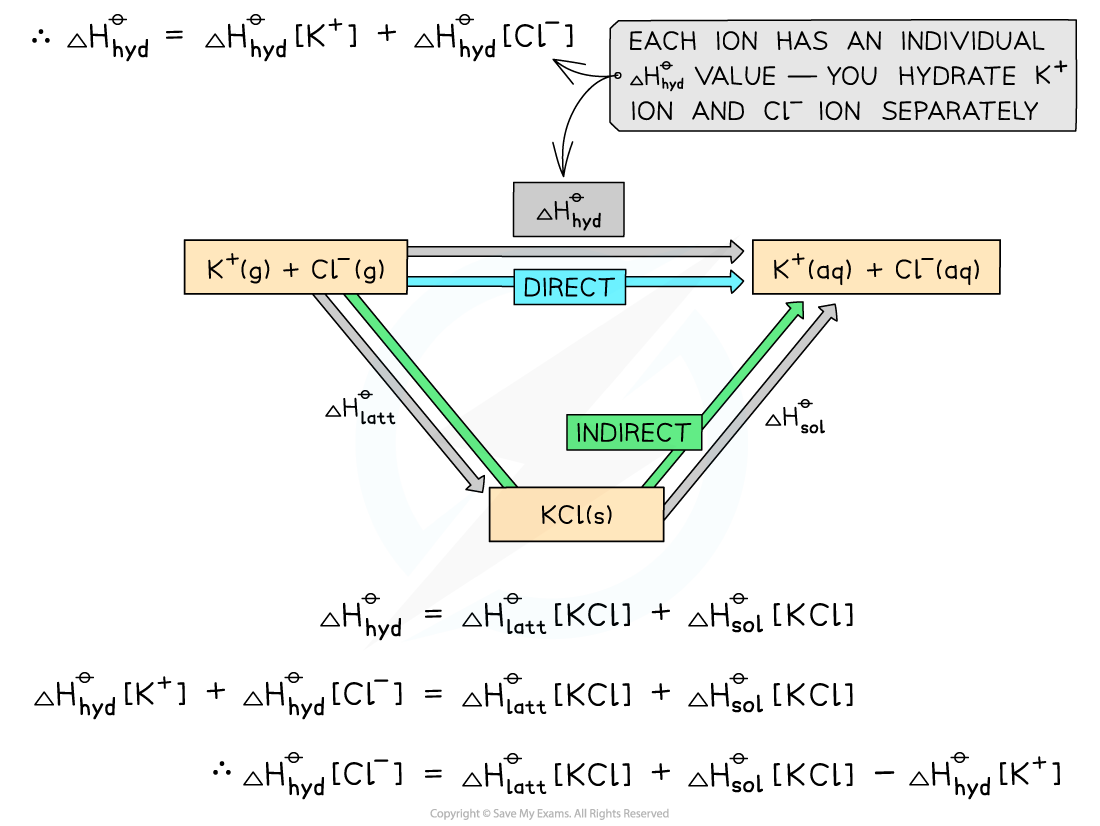
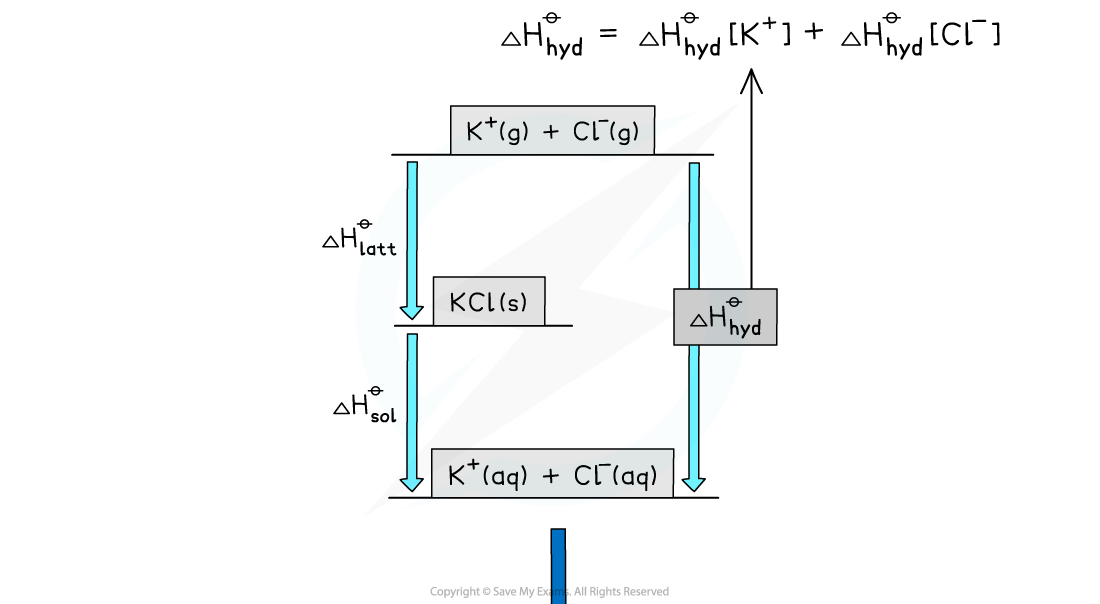
Worked example: Constructing an energy cycle and energy level diagram of KCl

Answer
Energy cycle:
Energy level diagram:
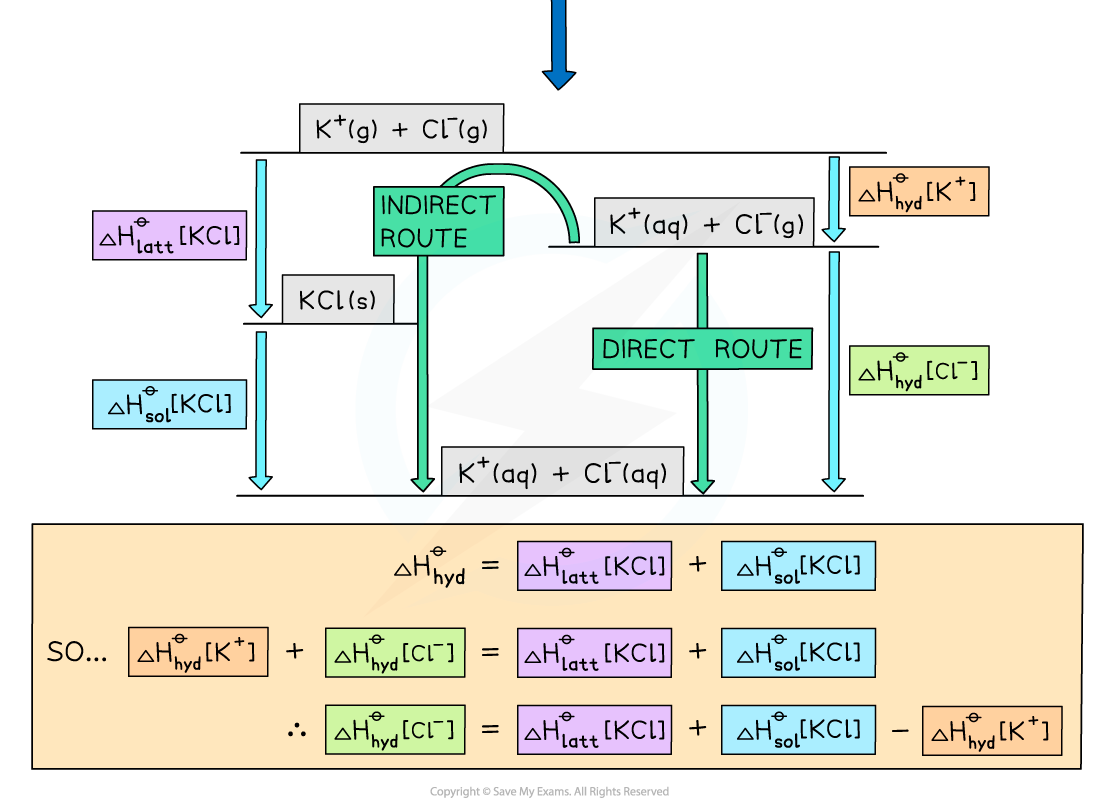
Worked example: Constructing an energy cycle and energy level diagram of MgCl2

Answer
Energy cycle:
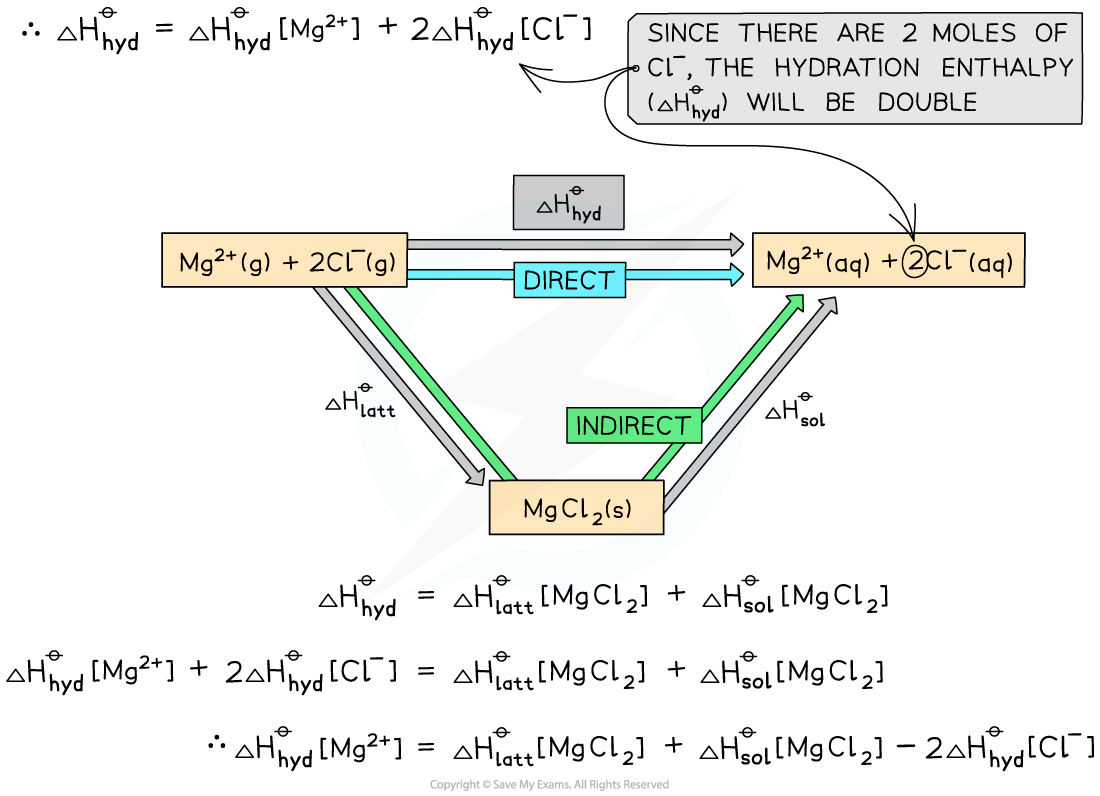
Energy level diagram:
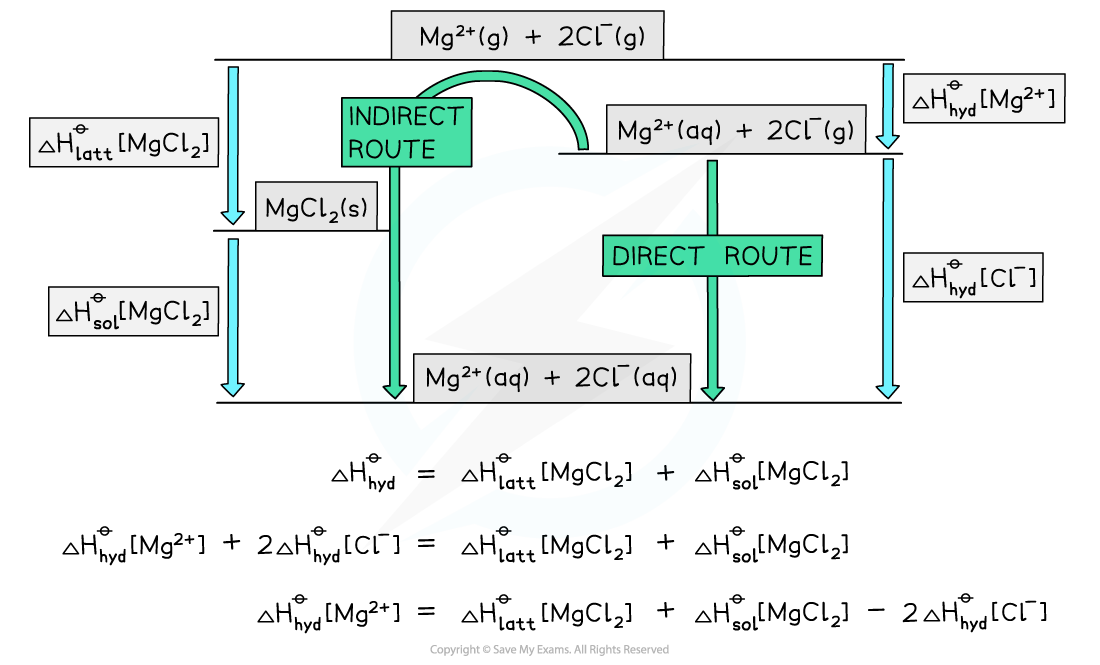
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









