- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记2.1.3 Period 3 Oxides
Period 3: Oxides
Reactions with oxygen & chlorine
Reaction of Period 3 elements with oxygen table
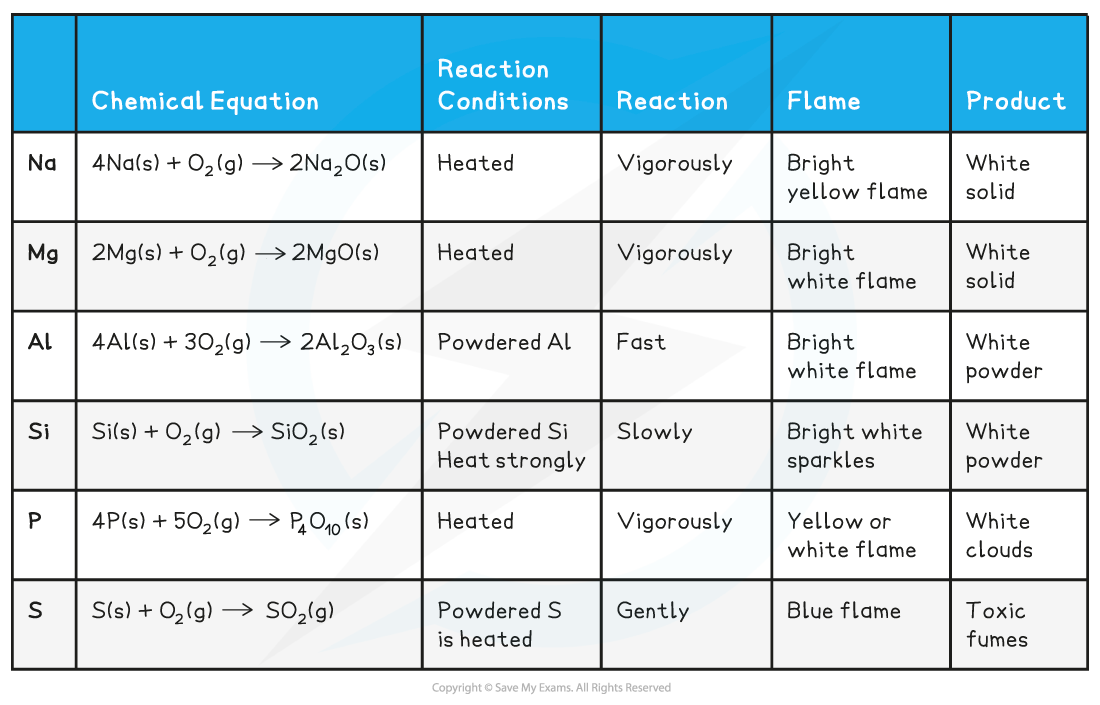
Reaction of Period 3 elements with chlorine table
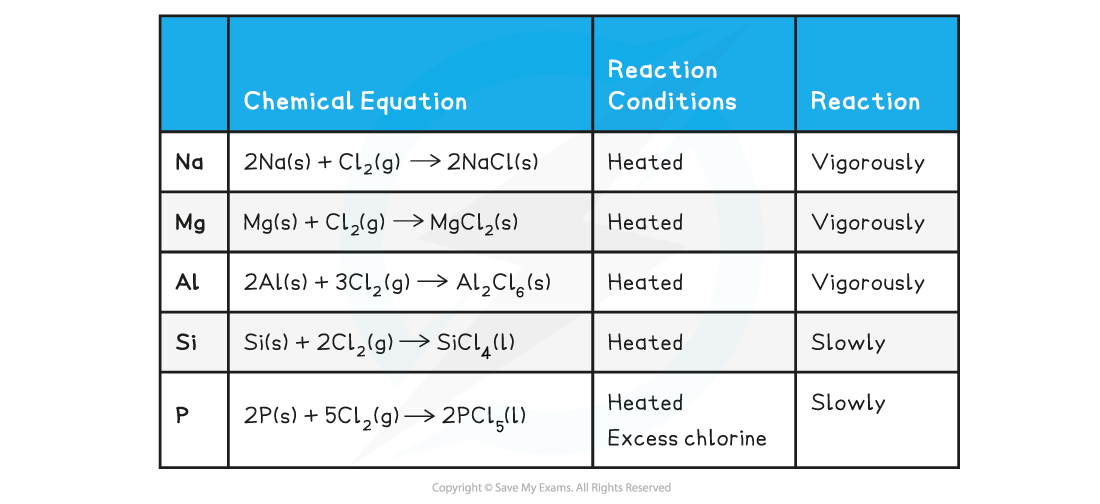
Reaction of sodium & magnesium with water
- Sodium reacts vigorously with cold water:
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
- The sodium melts into a ball and moves across the water surface until it disappears
- Hydrogen gas is given off
- The solution formed is strongly alkaline (pH 14) due to the sodium hydroxide which is formed
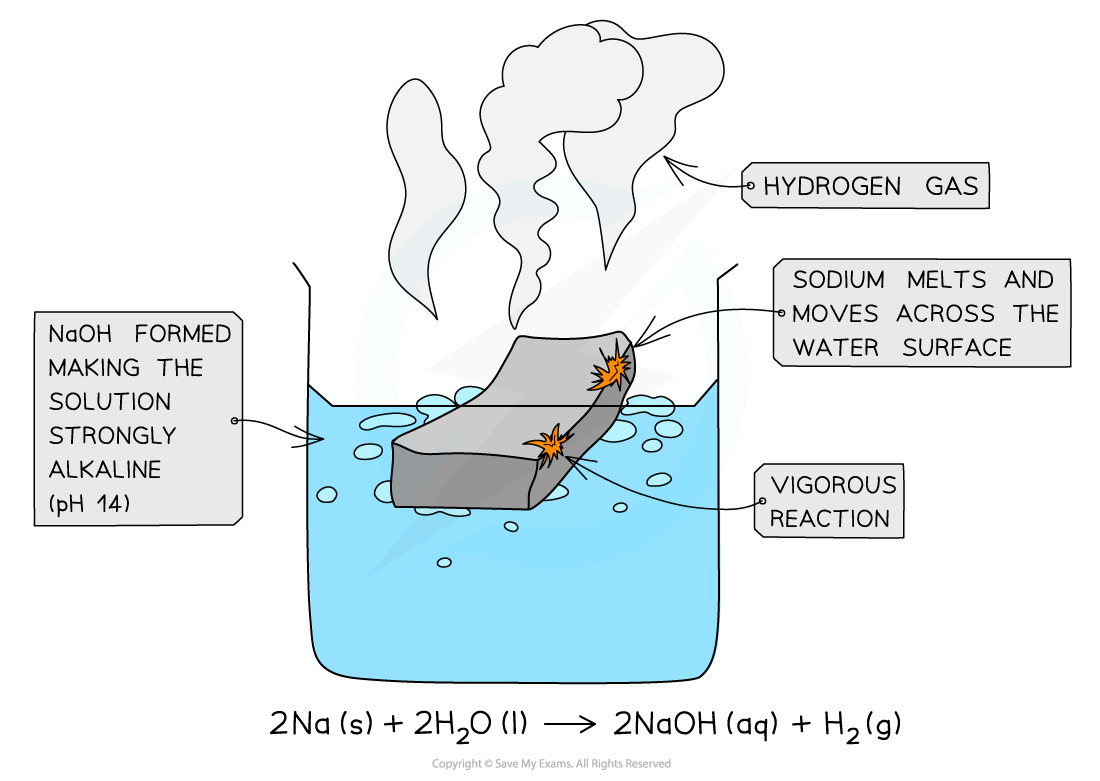
The diagram shows the reaction of sodium with cold water
- Magnesium reacts extremely slowly with cold water:
Mg(s) + 2H2O(l) → Mg(OH)2(aq) + H2(g)
- The solution formed is weakly alkaline (pH 11) as the formed magnesium hydroxide is only slightly soluble
- When magnesium is heated, it reacts vigorously with steam (water) to make magnesium oxide and hydrogen gas:
Mg(s) + H2O(g) → MgO(s) + H2(g)
Oxidation Number of the Period 3 Oxides
- Oxygen is more electronegative than any of the Period 3 elements
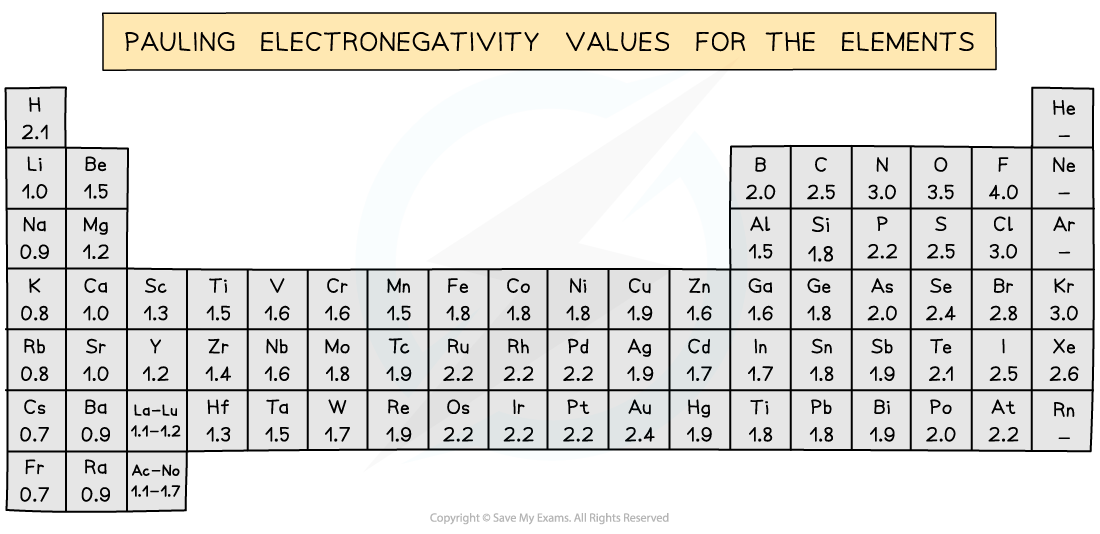
The Pauling scale shows the electronegativities of the elements in the periodic table. Oxygen has a higher electronegativity than any of the Period 3 elements which is why the Period 3 elements will have positive oxidation states and the oxygen a negative oxidation state in the oxides of Period 3 elements
- The Period 3 elements therefore have positive oxidation states in their oxides and the oxygen has a negative oxidation state of -2
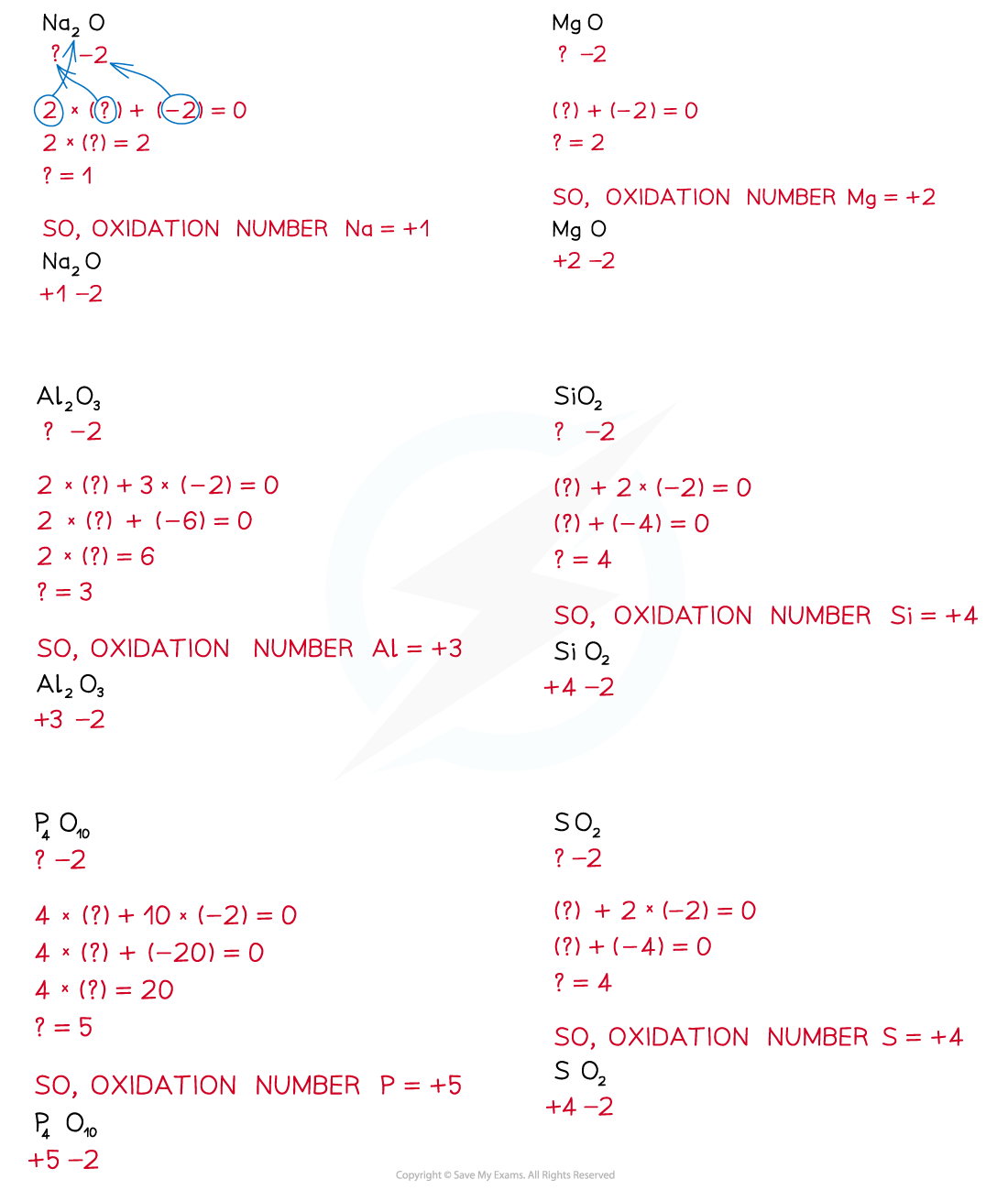
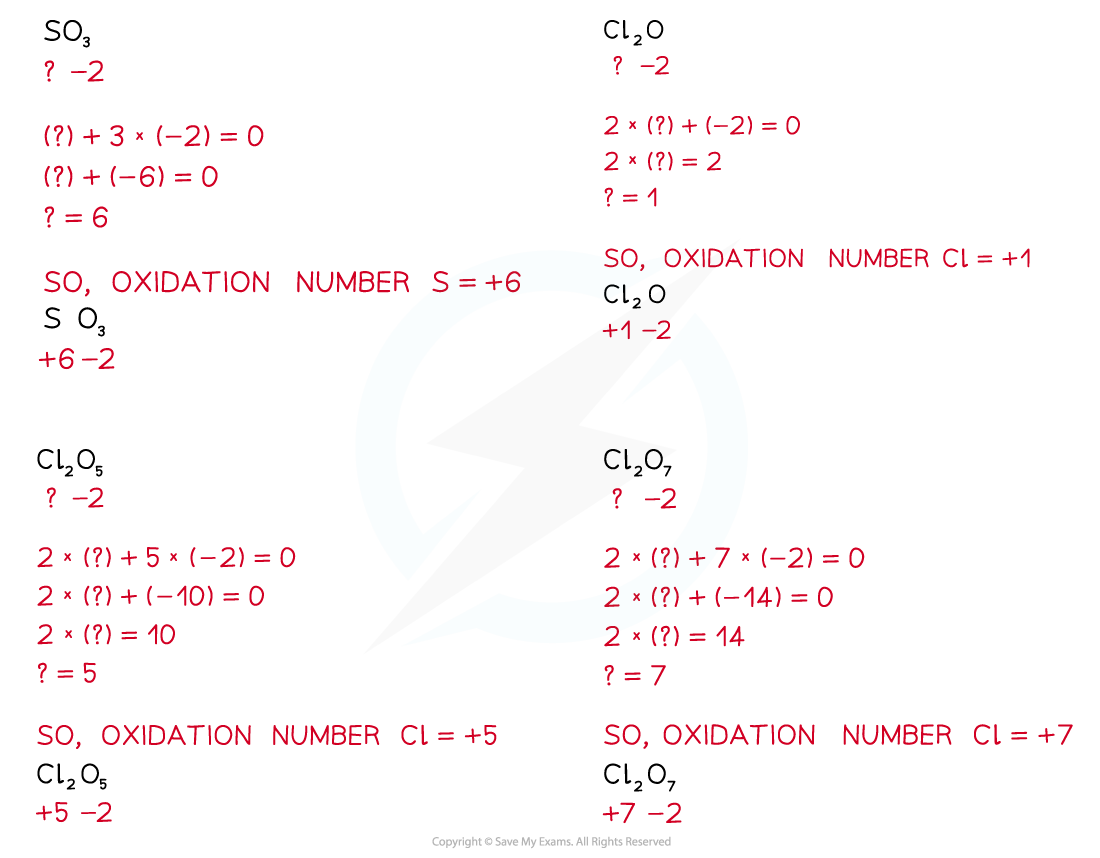
Oxidation states of the Period 3 elements in their oxides
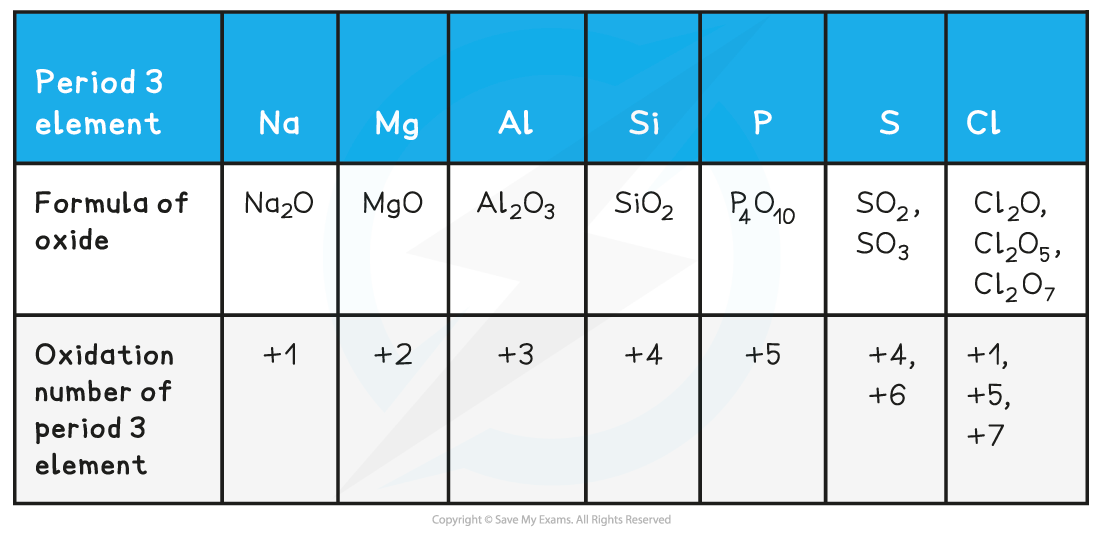
Formulae of the oxides of the Period 3 elements & their oxidation states table
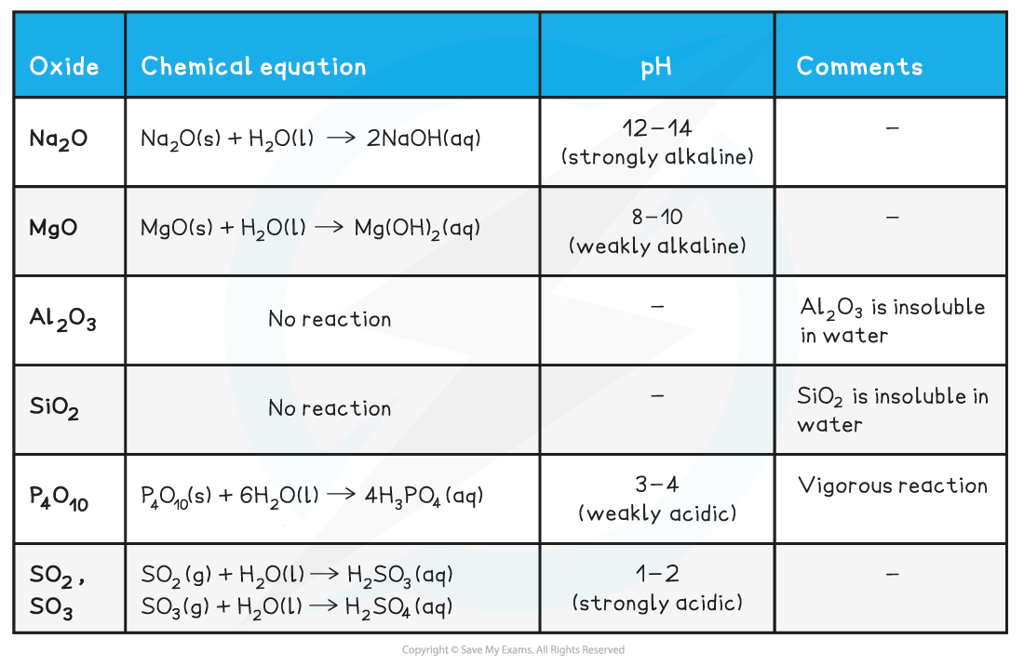
Reaction of Period 3 Oxides & Water
- Not all Period 3 oxides react with or are soluble in water
Reaction of Period 3 oxides with water table
Exam Tip
Since aluminium oxide does not react or dissolve in water, the oxide layer protects the aluminium metal from corrosion.
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









