- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记4.3.4 Metallic Bonding
Metallic Bonding
- Metal atoms are tightly packed together in lattice structures
- When the metal atoms are in lattice structures, the electrons in their outer shells are free to move throughout the structure
- The free-moving electrons are called ‘delocalised' electrons and they are not bound to their atom
- When the electrons are delocalised, the metal atoms become positively charged
- The positive charges repel each other and keep the neatly arranged lattice in place
- There are very strong electrostatic forces between the positive metal centres and the ‘sea’ of delocalised electrons
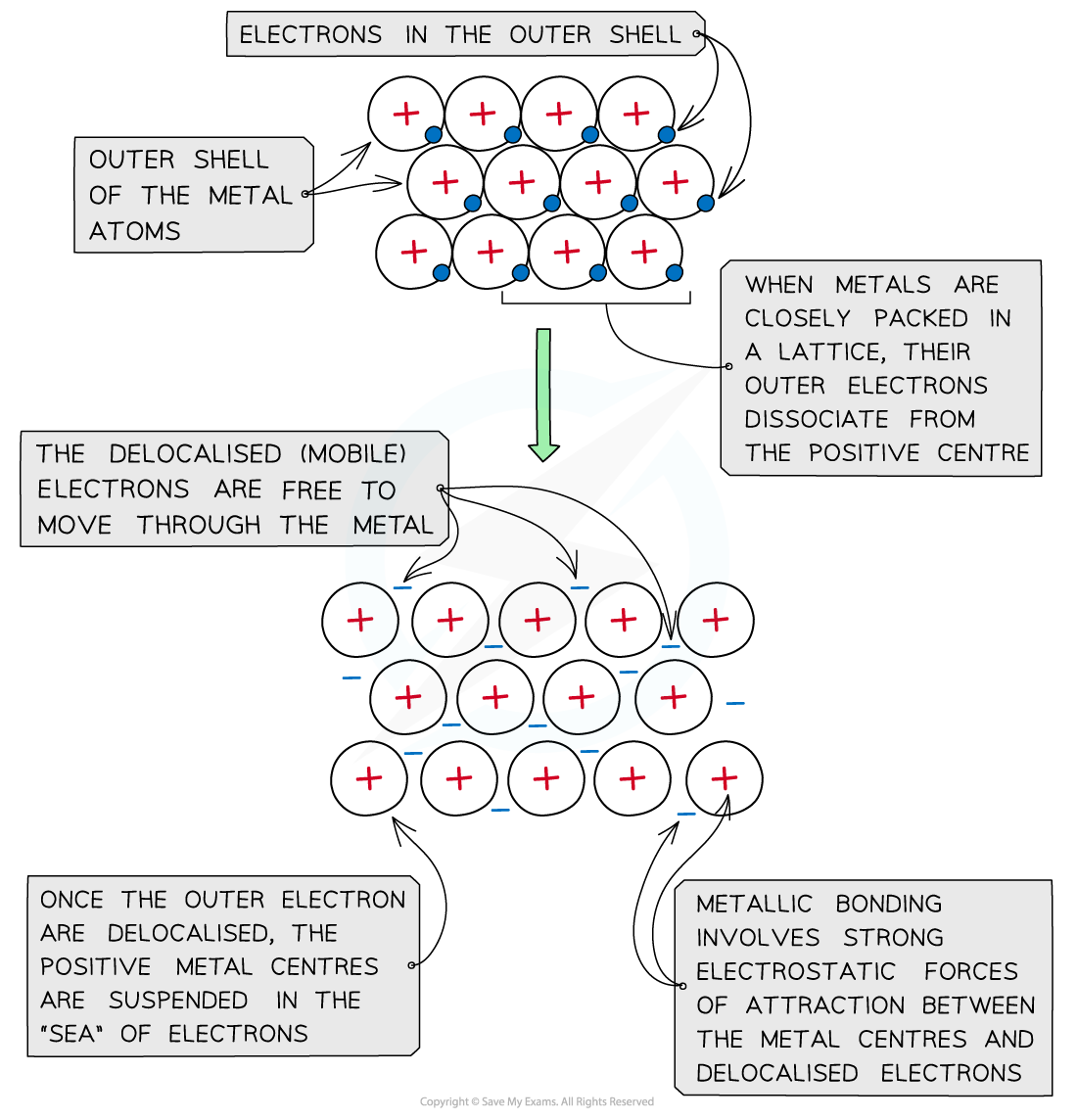
The positive metal centres are suspended in a ‘sea’ of delocalised electrons
Properties of Metals
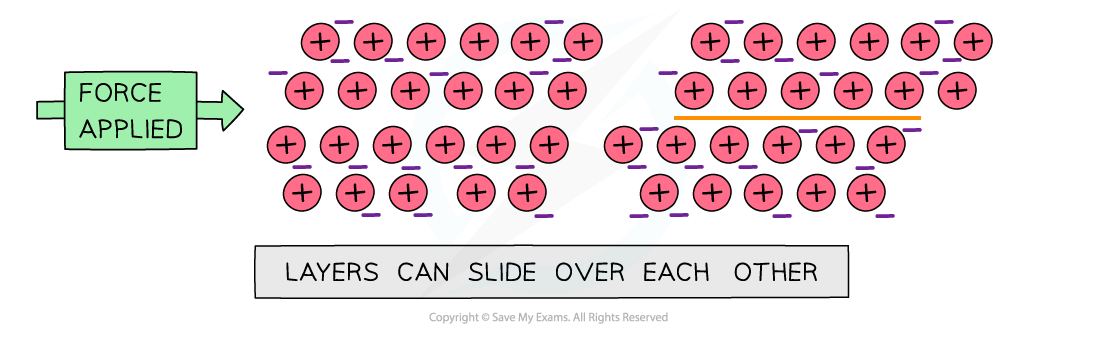
- Metallic compounds are malleable
- When a force is applied, the metal layers can slide
- The attractive forces between the metal ions and electrons act in all directions
- So when the layers slide, the metallic bonds are re-formed
- The lattice is not broken and has changed shape
- Metallic compounds are strong and hard
- Due to the strong attractive forces between the metal ions and delocalised electrons
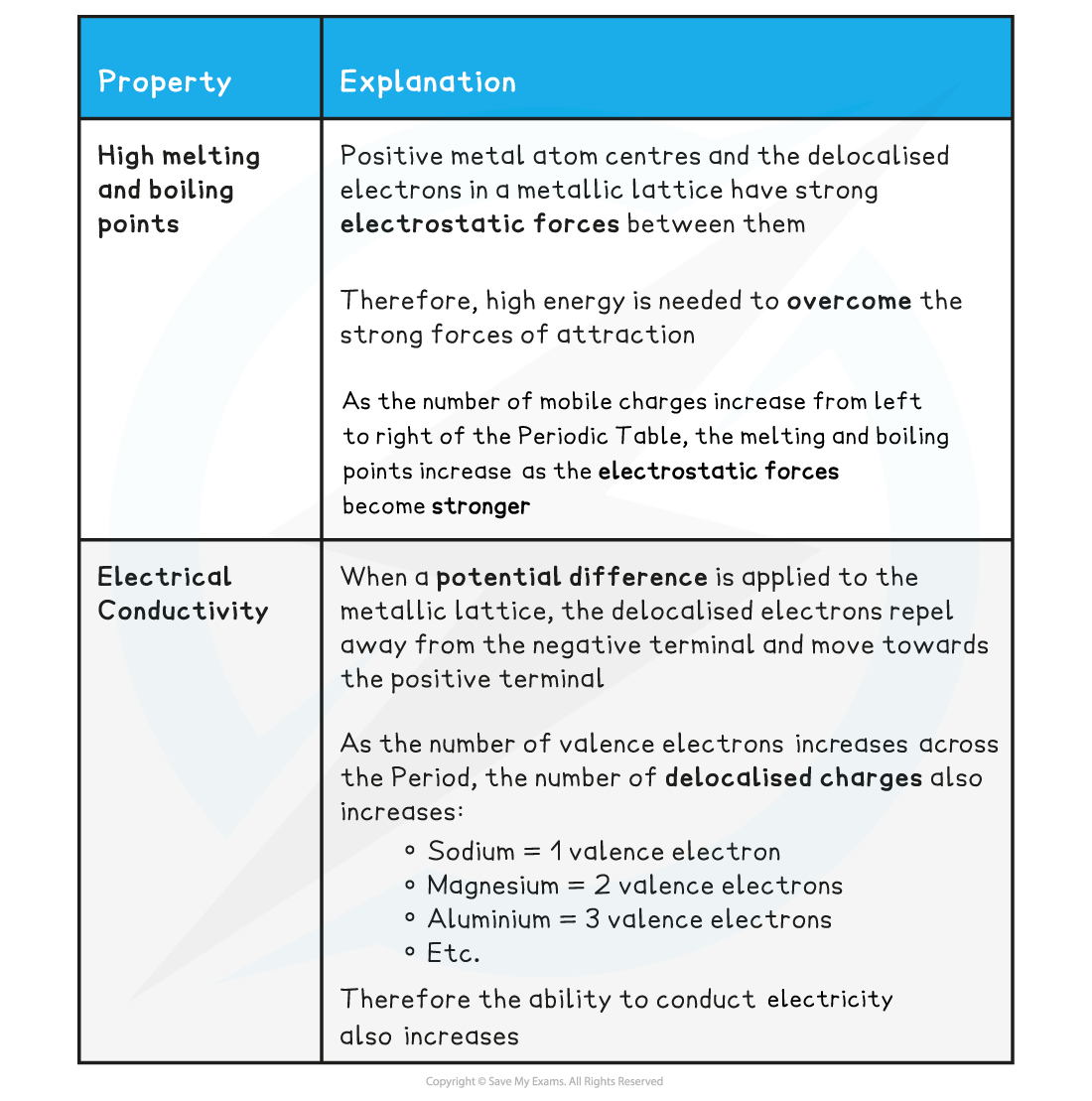
- Metals have high melting and boiling points
Conductivity
- Metals can conduct electricity when in the solid or liquid state
- As both in the solid and liquid state there are mobile electrons which can freely move around and conduct electricity
- Since the bonding in metals is non-directional, it does not really matter how the cations are oriented relative to each other
- The metal cations can be moved around and there will still be delocalised electrons available to hold the cations together
Metallic Bonding Summary Table
Strength of Metallic Bonds
Not all metallic bonds are equal. There are several factors that affect the strength of a metallic bond:
The charge on the metal ion
- The greater the charge on the metal ion, the greater the number of electrons in the sea of delocalised electrons and the greater the charge difference between the ions and the electrons
- A greater charge difference leads to a stronger electrostatic attraction, and therefore a stronger metallic bond
- This effect can be seen in melting point data across a period, as the charge on the metal ion increases without a significant change in ionic radius:
| Group | 1 | 2 | 3 (13) |
| Metal | Sodium | Magnesium | Aluminium |
| Melting point / K | 371 | 923 | 933 |
The radius of the metal ion
- Metal ions with smaller ionic radii exert a greater attraction on the sea of delocalised electrons
- This greater attraction means a stronger metallic bond, requiring more energy to break
- This can be seen in data from metals, descending a group, where the charge on the ion remains constant but the ionic radius increases:
| Period | 1 | 2 | 3 |
| Metal | Sodium | Potassium | Rubidium |
| Melting point / K | 371 | 336 | 312 |
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









