Hydrogen
- The hydrogen atom has only one s orbital
- The s orbitals of the two hydrogen atoms will overlap to form a σ bond

Direct overlap of the 1s orbitals of the hydrogen atoms results in the formation of a σ bond

Sigma orbitals can be formed from the end-on overlap of s orbitals

Hydrogen fluoride has sigma bonds between s and p orbitals

Fluorine has sigma bonds between p orbitals

π orbitals are formed by the end-on overlap of p orbitals
Hydrogen

Direct overlap of the 1s orbitals of the hydrogen atoms results in the formation of a σ bond
Ethene

Overlap of the p orbitals results in the forming of a π bond in ethene

Each carbon atom in ethene forms two sigma bonds with hydrogen atoms and one σ bond with another carbon atom. The fourth electron is used to form a π bond between the two carbon atoms
Ethyne
 Ethyne has a triple bond formed from two π bonds and one σ bond between the two carbon atoms
Ethyne has a triple bond formed from two π bonds and one σ bond between the two carbon atomsWhat type of molecular orbitals are found in nitrogen, N2, and hydrogen cyanide, HCN?
Answer
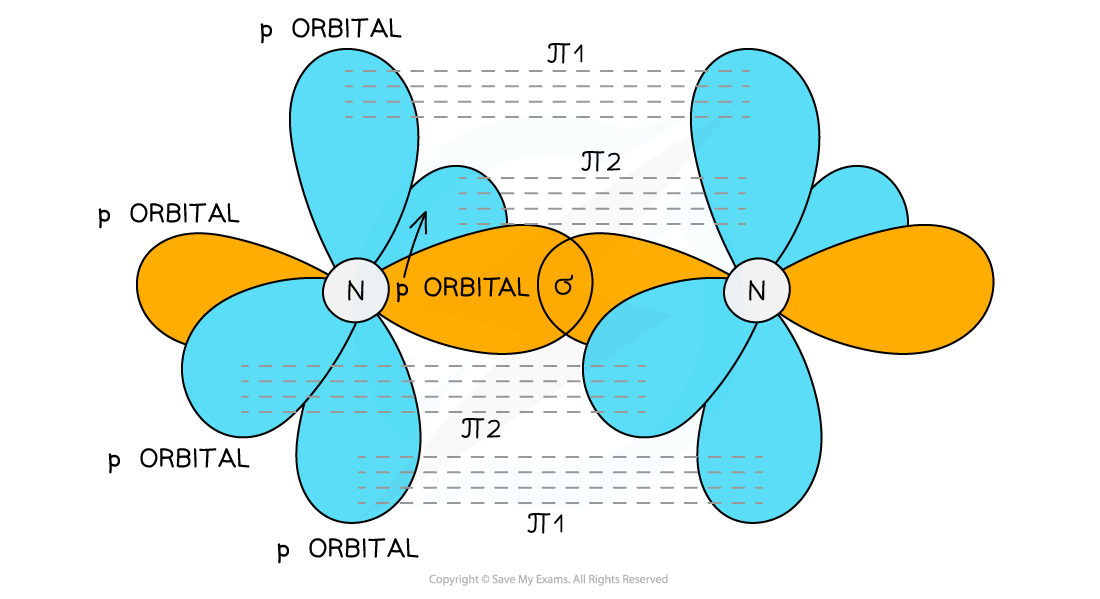 The triple bond is formed from two π bonds and one σ bond
The triple bond is formed from two π bonds and one σ bond
 Hydrogen cyanide has a triple bond formed from a σ bond and the overlap of two sets of p orbitals of nitrogen
Hydrogen cyanide has a triple bond formed from a σ bond and the overlap of two sets of p orbitals of nitrogen
转载自savemyexams

© 2026. All Rights Reserved. 沪ICP备2023009024号-1