- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记7.5.2 Basic Properties
Basic Properties
- The nitrogen atom in ammonia and amine molecules can accept a proton (H+ ion)
- They can therefore act as bases in aqueous solutions by donating its lone pair of electrons to a proton and form a dative bond
- For example, ammonia undergoes an acid-base reaction with dilute hydrochloric acid (HCl) to form a salt
NH3 + HCl → NH4+Cl-
base acid salt
- Amines react with HCl to form amine salts, such as ethylammonium chloride
C2H5NH2 + HCl → C2H5NH3+Cl-
base acid salt
- Like ammonium salts, amine salts are soluble ionic compounds, so when the alkyl group is small they are water soluble but become less so as the carbon chain increases
- We can show the reaction in Brønsted-Lowry terms, where ammonia or the amine acts as a proton acceptor:
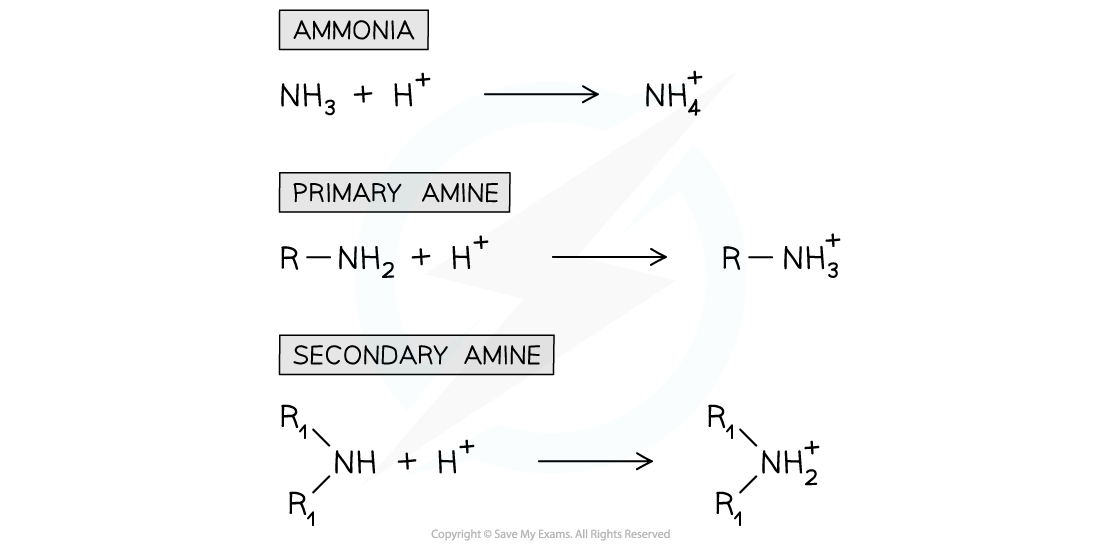
The nitrogen atom in ammonia and amines can donate its lone pair of electrons to form a bond with a proton and therefore act as a base
Strength of ammonia and amines as bases
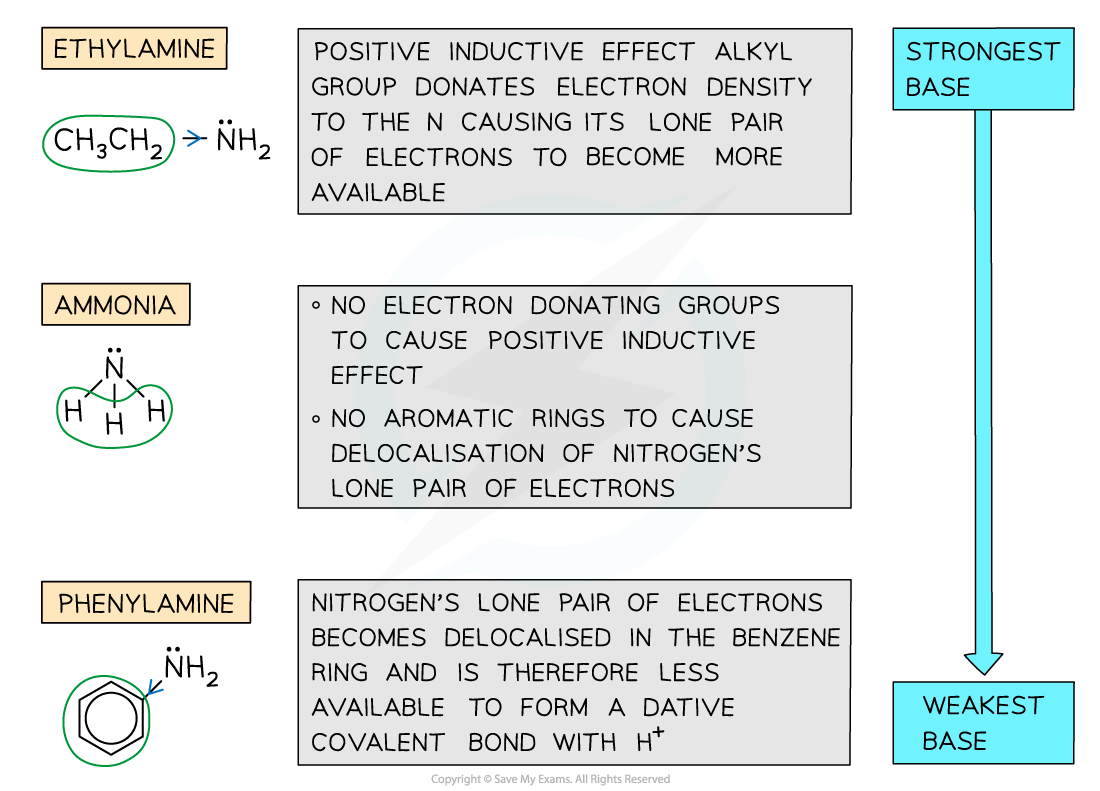
- The strength of amines depends on the ability of the lone pair of electrons on the nitrogen atom to accept a proton and form a dative covalent bond
- The more readily a proton is attracted, the stronger the base is
- Factors that may affect the basicity of amines include:
- Positive inductive effect - Some groups such as alkyl groups donate electron density to the nitrogen atom causing the lone pair of electrons to become more available and therefore increasing the amine’s basicity
- Delocalisation - The presence of aromatic rings such as the benzene ring causes the lone pair of electrons on the nitrogen atom to be delocalised into the benzene ring
- The lone pair becomes less available to form a dative covalent bond with ammonia and hence decreases the amine’s basicity
- For example, ethylamine (which has an electron-donating ethyl group) is more basic than phenylamine (which has an electron-withdrawing benzene ring)

Ethylamine is more basic than phenylamine due to electron donating ethyl group which increases electron density on the nitrogen and makes it more attractive to protons
Exam Tip
Amines have a fishy smell, which disappears as soon as you add an acid, because you are making an non-volatile salt. Decomposing fish give off amines which are produced by the bacterial decomposition of proteins.
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









