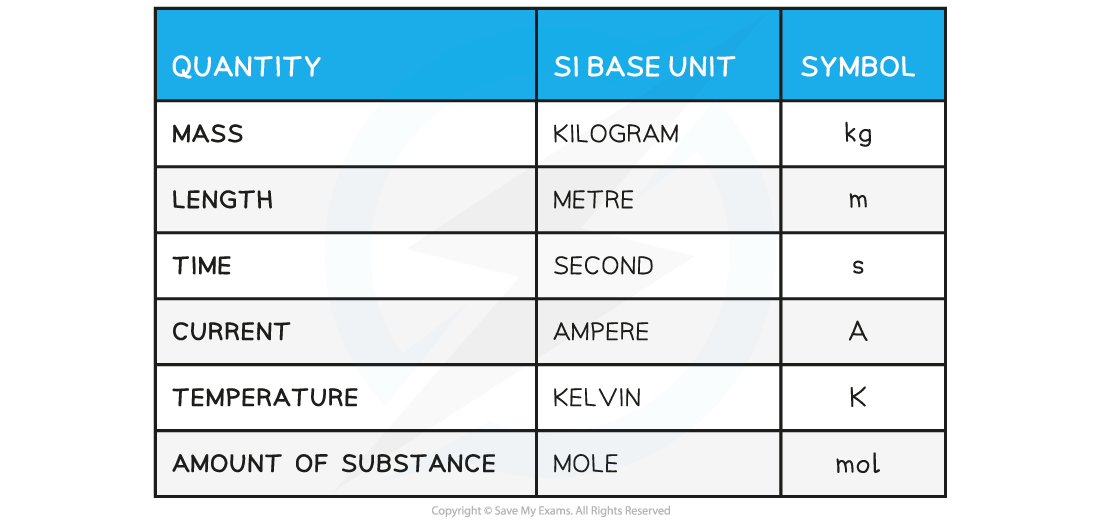
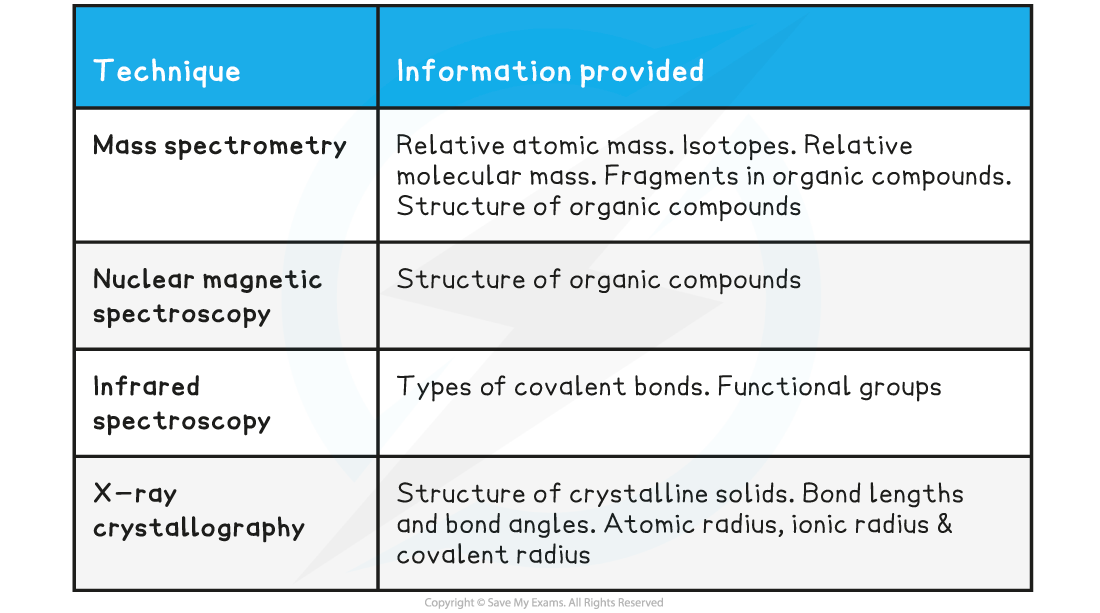
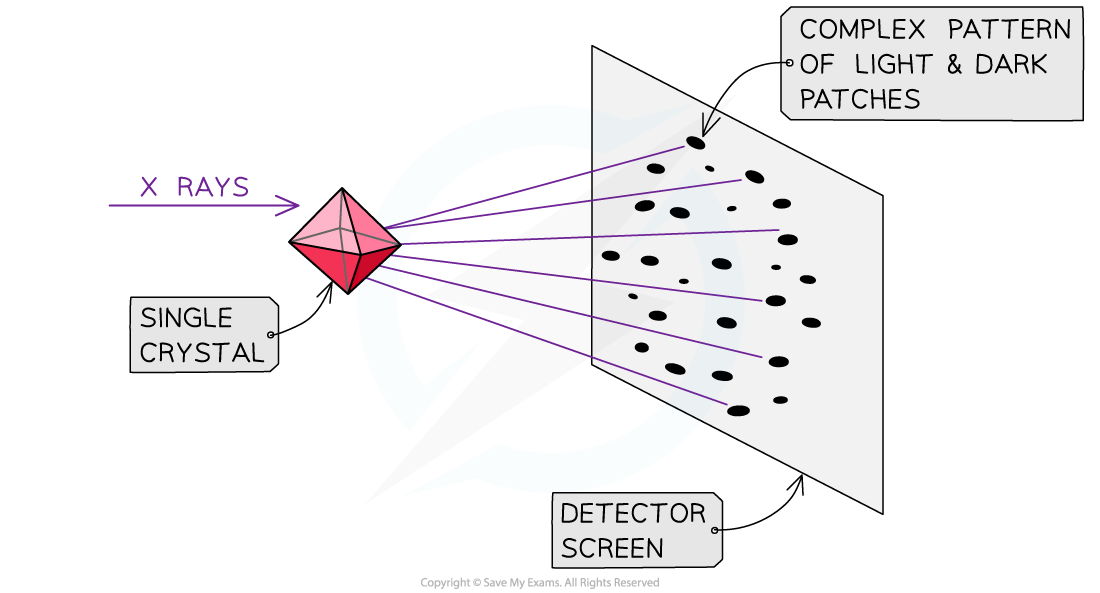
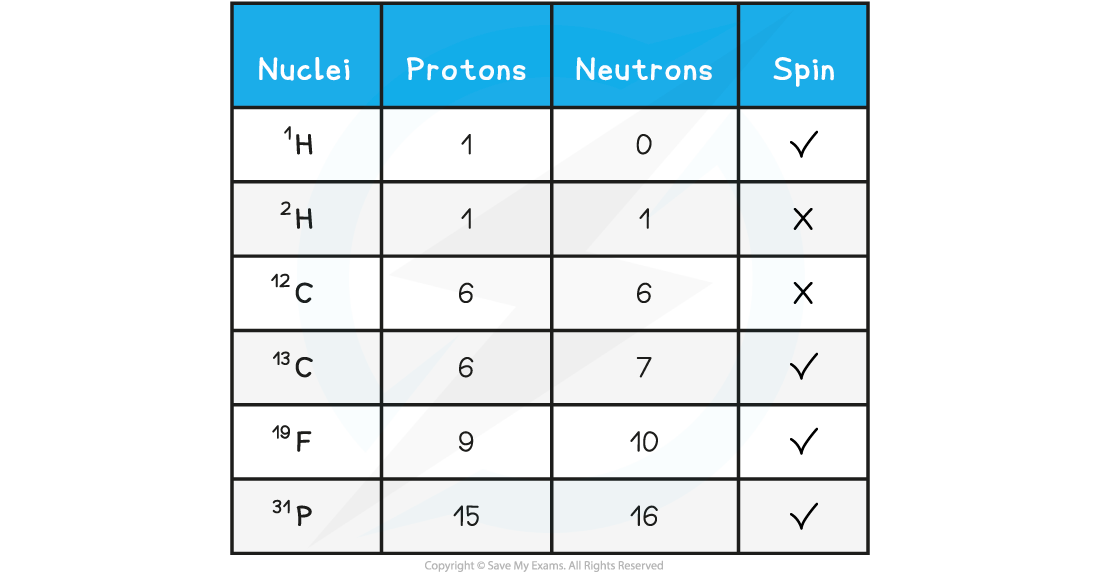
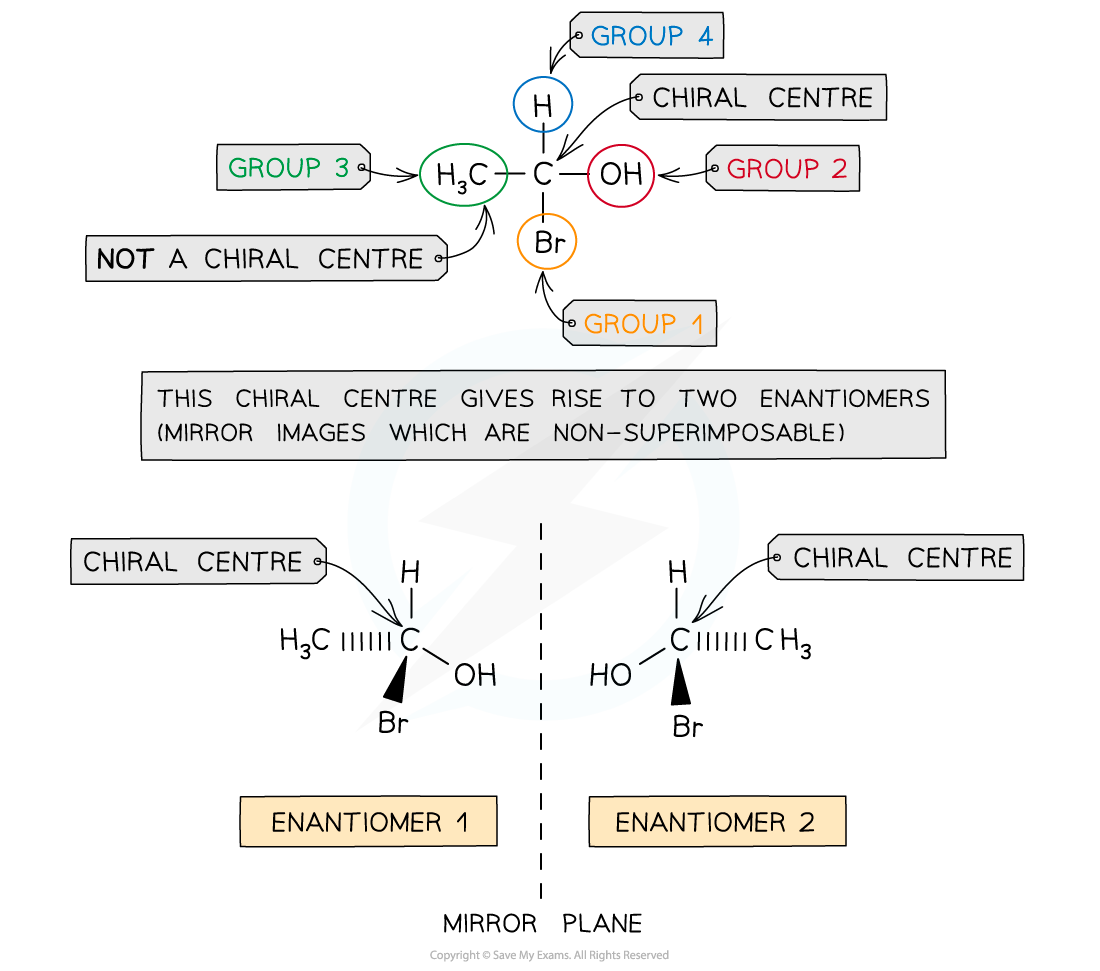
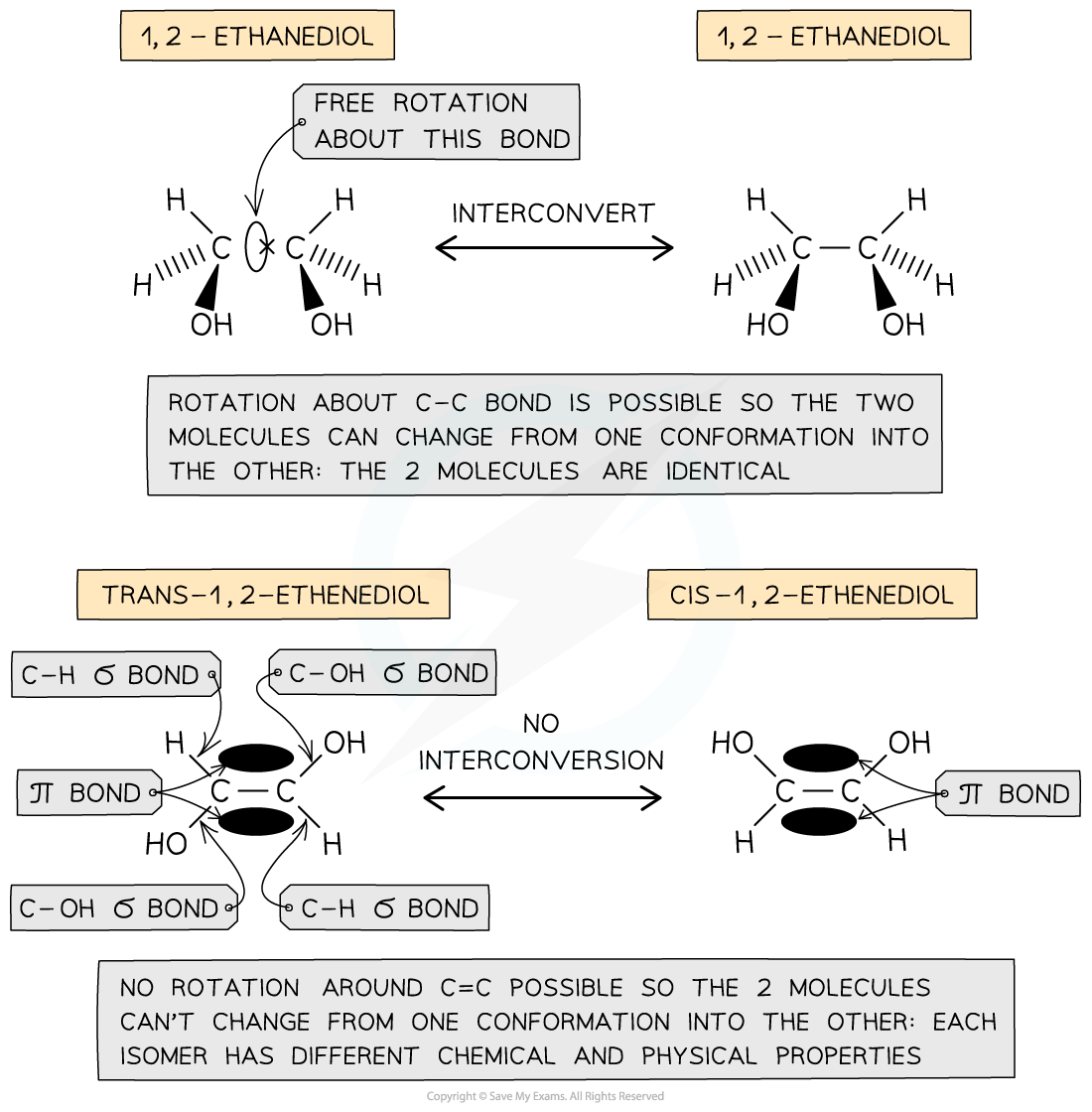
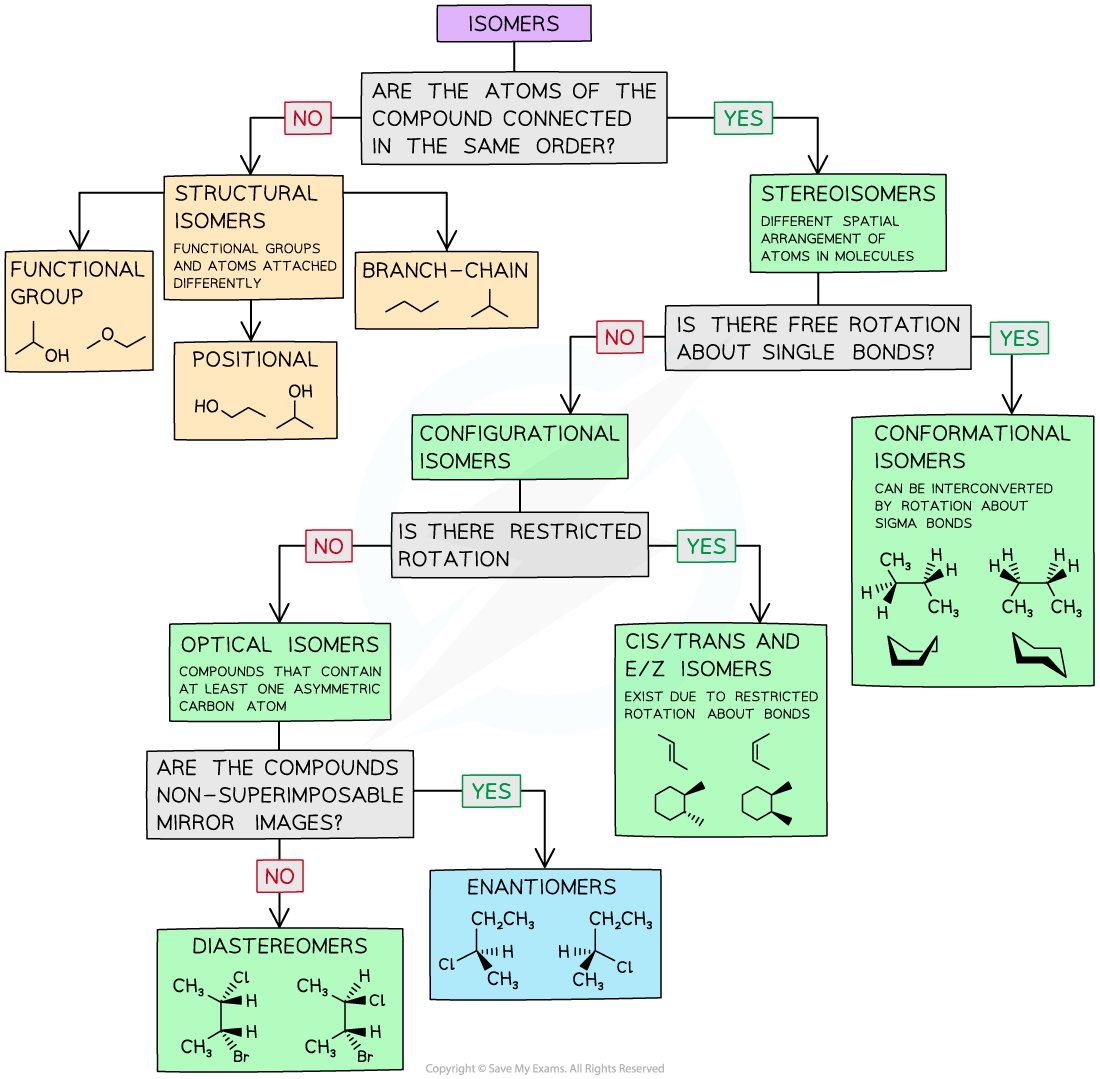
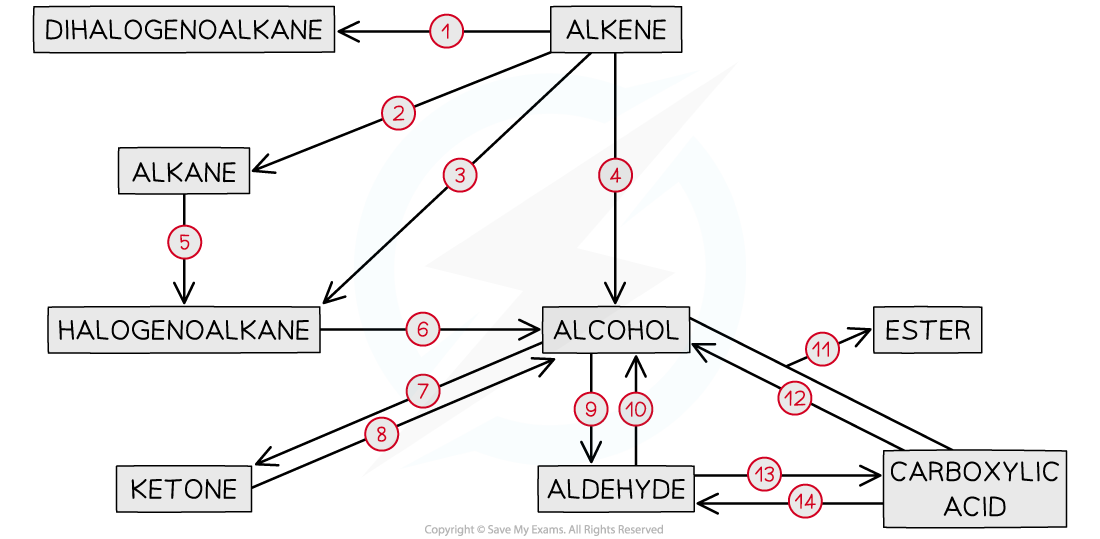
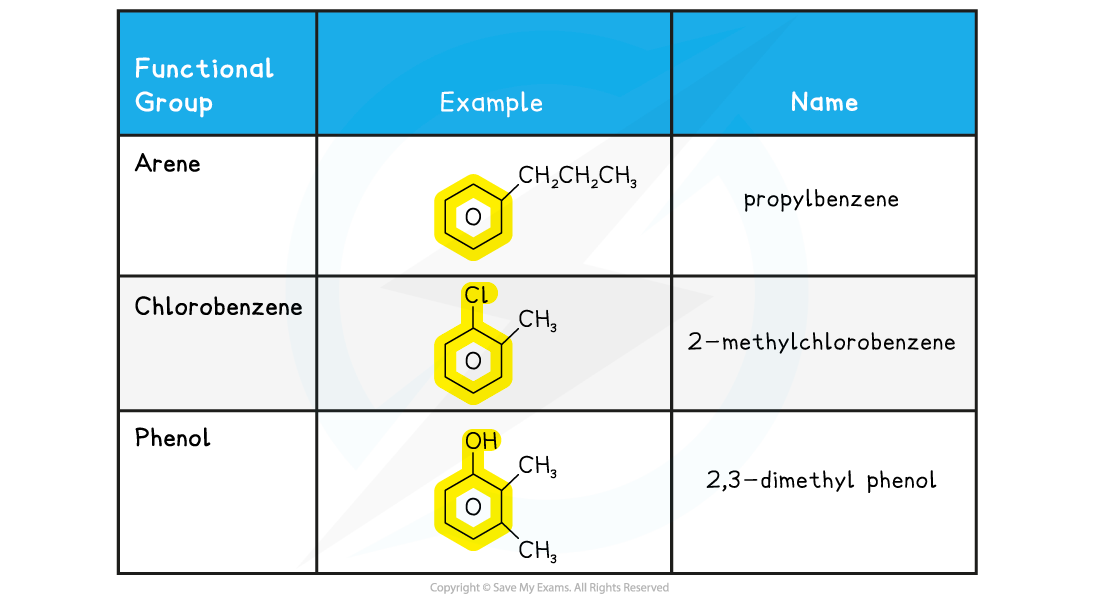
IB DP Physics: HL复习笔记1.1.3 Estimating Physical Quantities
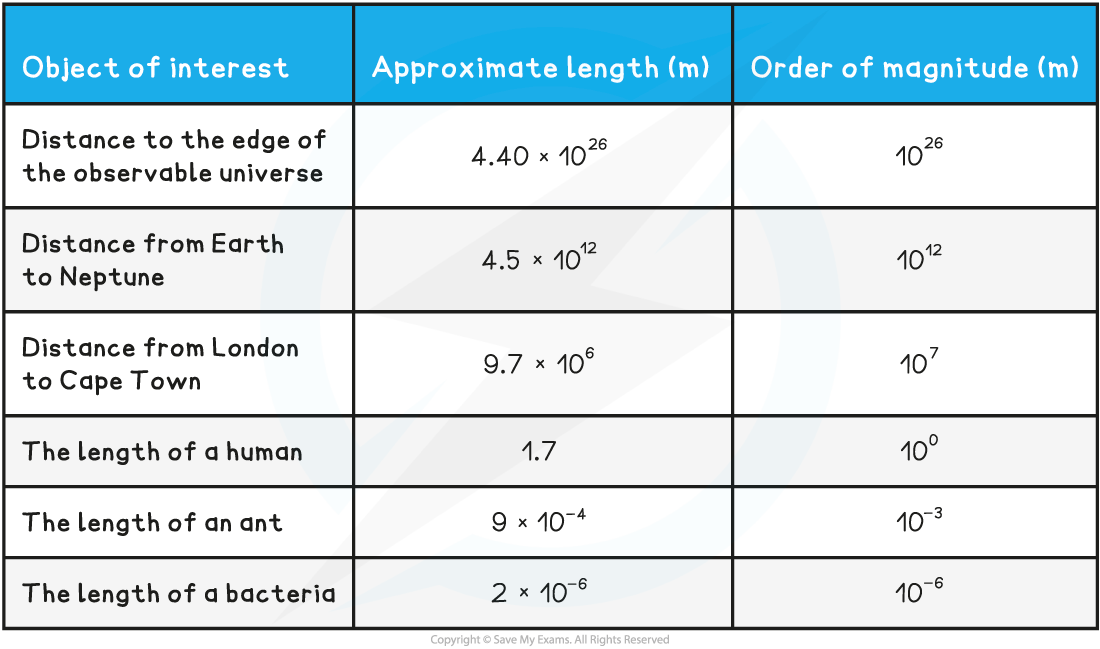
Orders of Magnitude When a number is expressed in an order of 10, this is an order of magnitude. Example: If a number is described as 3 × 108 then that number is actually 3 × 100 000 000 The order ...