CIE A Level Physics复习笔记8.2.2 Interference & Coherence
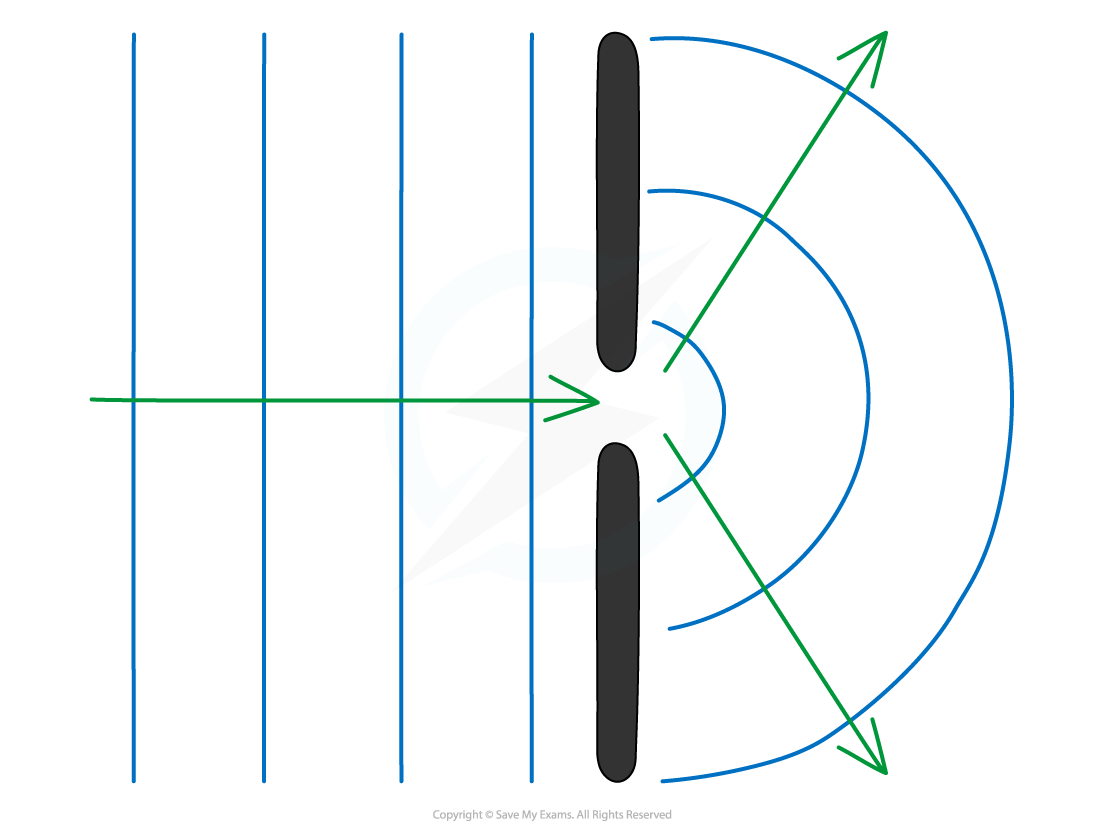
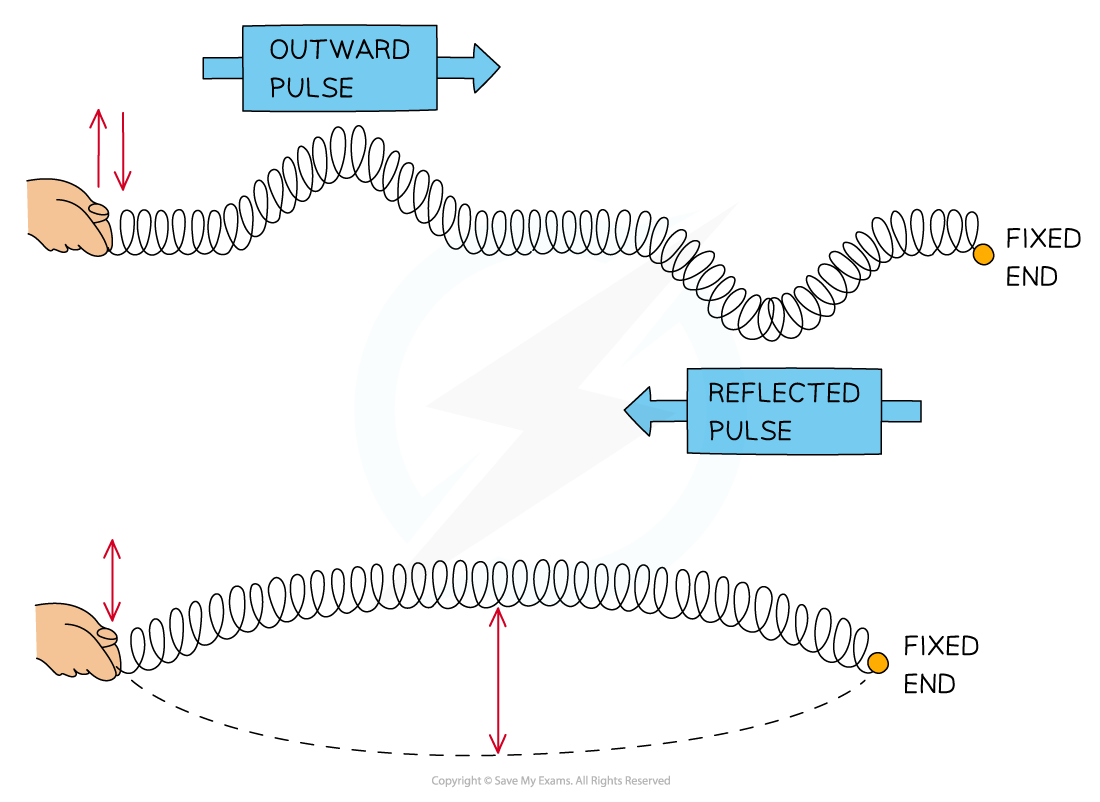
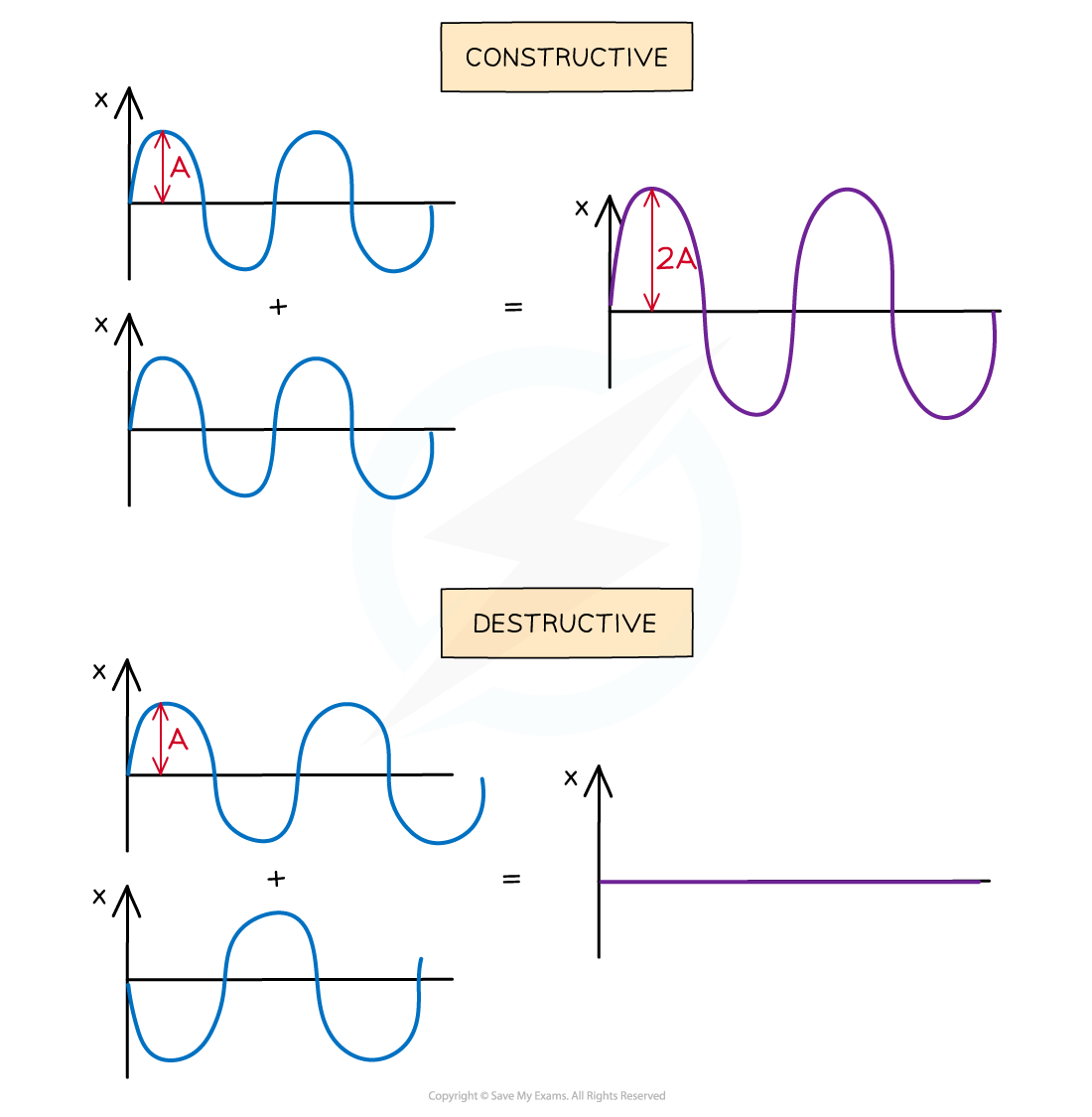
Interference & Coherence Interference occurs when waves overlap and their resultant displacement is the sum of the displacement of each wave This result is based on the principle of superpositi...