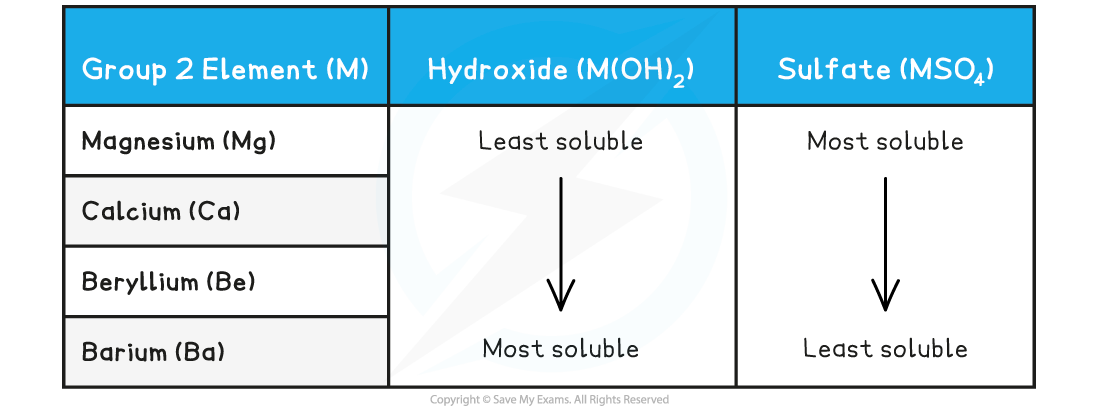
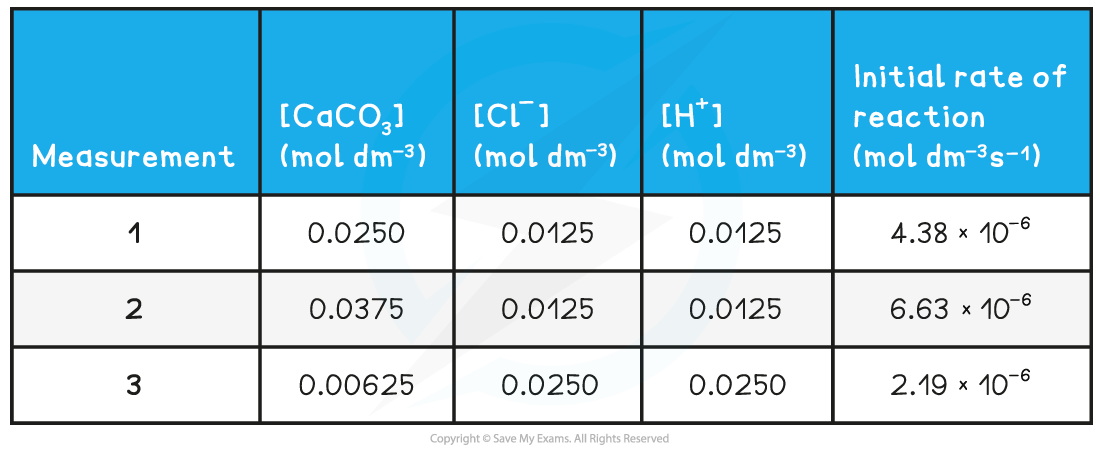
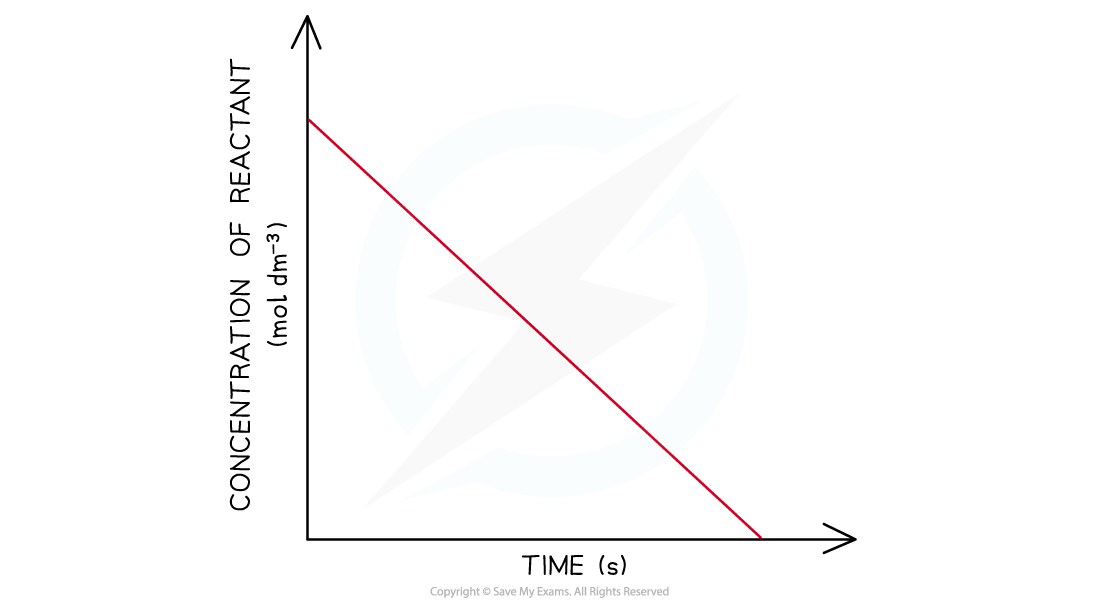
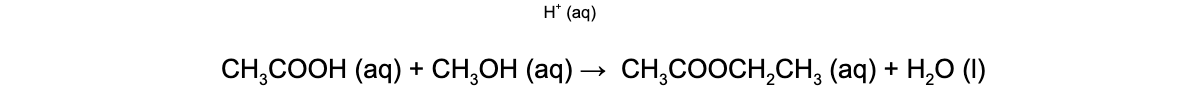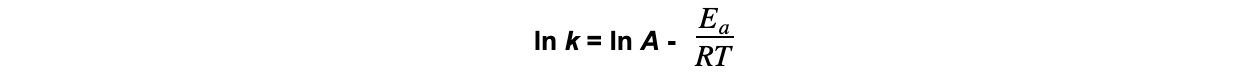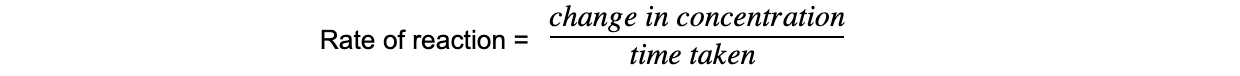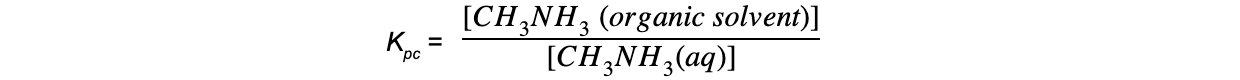CIE A Level Chemistry复习笔记6.1.1 Similarities, Trends & Compounds of Magnesium to Barium
Effect of Ionic Radius on Thermal Stability of Group 2 Nitrates & Carbonates The Group 2 nitrates and carbonates become more thermally stable going down the group The charge density of the cati...