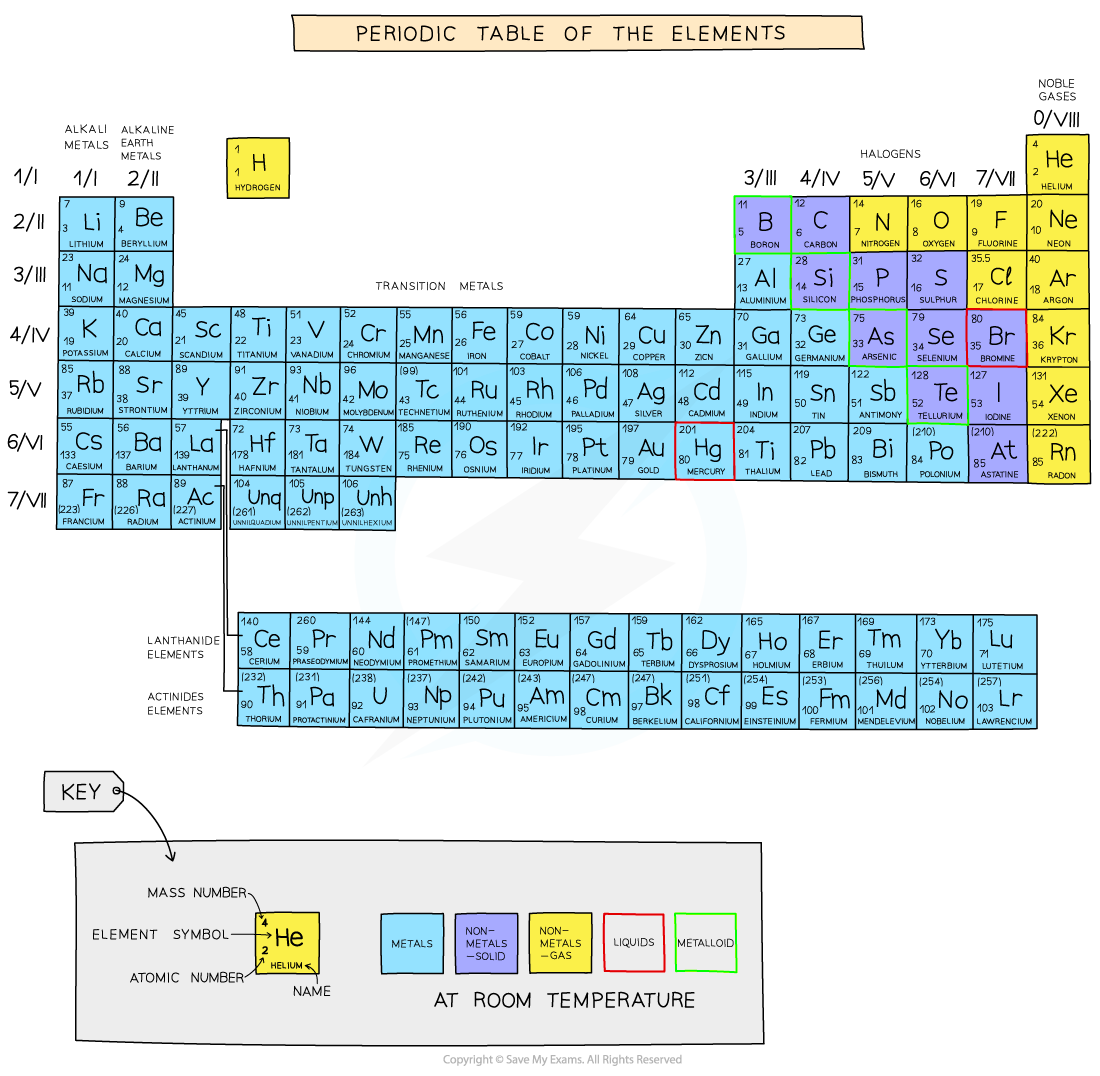
IB DP Chemistry: SL复习笔记3.1.2 Periodic Trends: Physical - Atomic & Ionic Radius
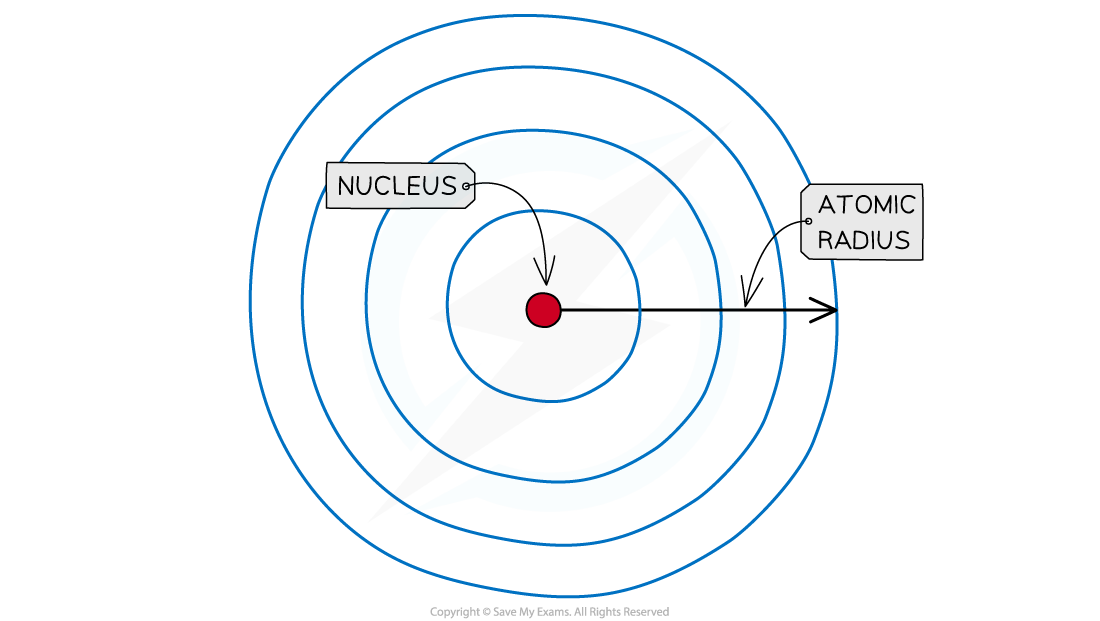
Atomic & Ionic Radius Atomic radius The atomic radius of an element is a measure of the size of an atom It is the distance between the nucleus of an atom and the outermost electron shell It can...