- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记3.2.1 Alkanes
Production of Alkanes: Hydrogenation & Cracking
- Alkanes are hydrocarbons that can be produced by the addition reaction of hydrogen to an alkene or by cracking of longer alkane chains
Production of alkanes from addition reactions
- Alkenes are unsaturated organic molecules and contain C-C double bonds
- When hydrogen gas and an alkene are heated and passed over a finely divided Pt/Ni catalyst, the addition reaction produces an alkane:
- The Pt/Ni catalyst is finely divided to increase its surface area and therefore increase the rate of reaction
- Eg. butane from 1-butene
 Hydrogen gas is added to 1-butene which are then heated and passed over a Pt/Ni catalyst to produce butane
Hydrogen gas is added to 1-butene which are then heated and passed over a Pt/Ni catalyst to produce butane
- The addition reaction of alkenes with hydrogen is called hydrogenation
- Hydrogenation is often used in the manufacture of margarine from vegetable oil
- Vegetable oil is an unsaturated organic molecule with many C-C double bonds
- When these are partially hydrogenated, their hydrocarbon chains become straighter
- This raises the melting point of the oils which is why margarine is a soft solid and vegetable oil a liquid at room temperature
Production of alkanes from cracking
- In cracking large, less useful hydrocarbon molecules found in crude oil are broken down into smaller, more useful molecules
- The large hydrocarbon molecules are fed into a steel chamber and heated to a high temperature and then passed over an aluminium oxide (Al2O3) catalyst
- The chamber does not contain any oxygen to prevent combustion of the hydrocarbon to water and carbon dioxide
- When a large hydrocarbon is cracked, a smaller alkane and alkene molecules are formed
- Eg. octane and ethene from decane
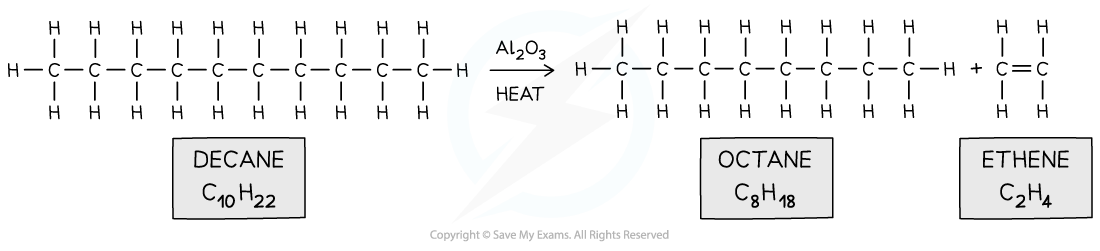 Long hydrocarbons are cracked by heating them and using aluminium oxide catalyst into smaller hydrocarbons and an alkene
Long hydrocarbons are cracked by heating them and using aluminium oxide catalyst into smaller hydrocarbons and an alkeneExam Tip
Remember that hydrogenation is an exothermic reaction and cracking is an endothermic reaction.
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









