- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 1.7.2 Covalent Bonds: Dot & Cross Diagrams
Edexcel IGCSE Chemistry 复习笔记 1.7.2 Covalent Bonds: Dot & Cross Diagrams
Covalent Bonds: Dot & Cross Diagrams
- Covalent substances tend to have small molecular structures, such as Cl2, H2O or CO2
- These small molecules are known as simple molecules
- Small covalent molecules can be represented by dot and cross diagrams
- You need to be able to describe and draw the structures of the following molecules using dot-and-cross diagrams: hydrogen (H2), chlorine (Cl2), oxygen (O2), nitrogen (N2), hydrogen chloride (HCl), water (H2O), ammonia (NH3) and methane (CH4)
- The correct dot and cross diagrams for these molecules are shown below:
Diatomic Molecules
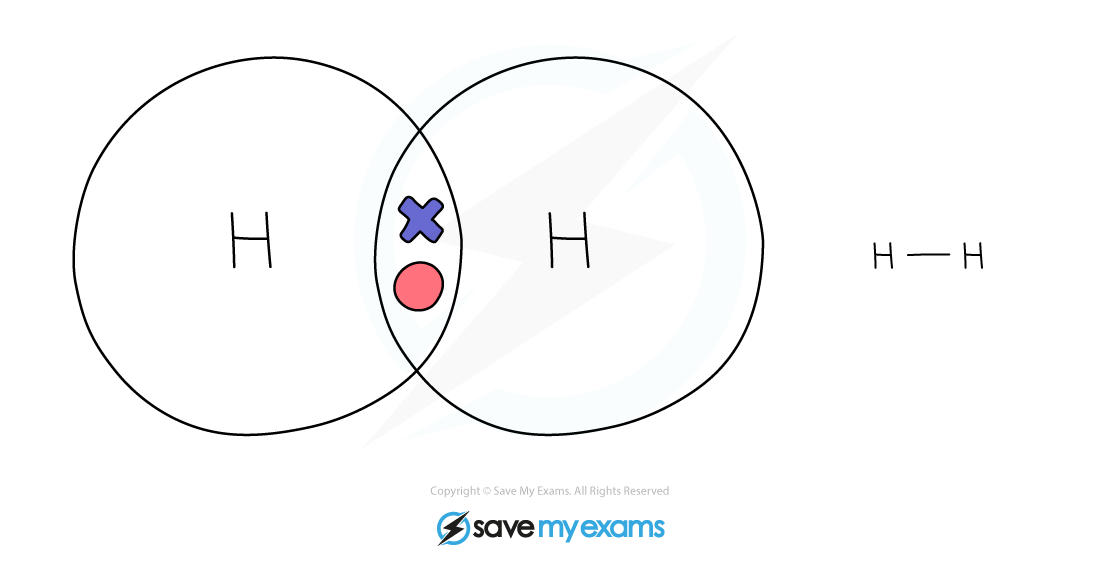
Dot & cross representation of a molecule of hydrogen
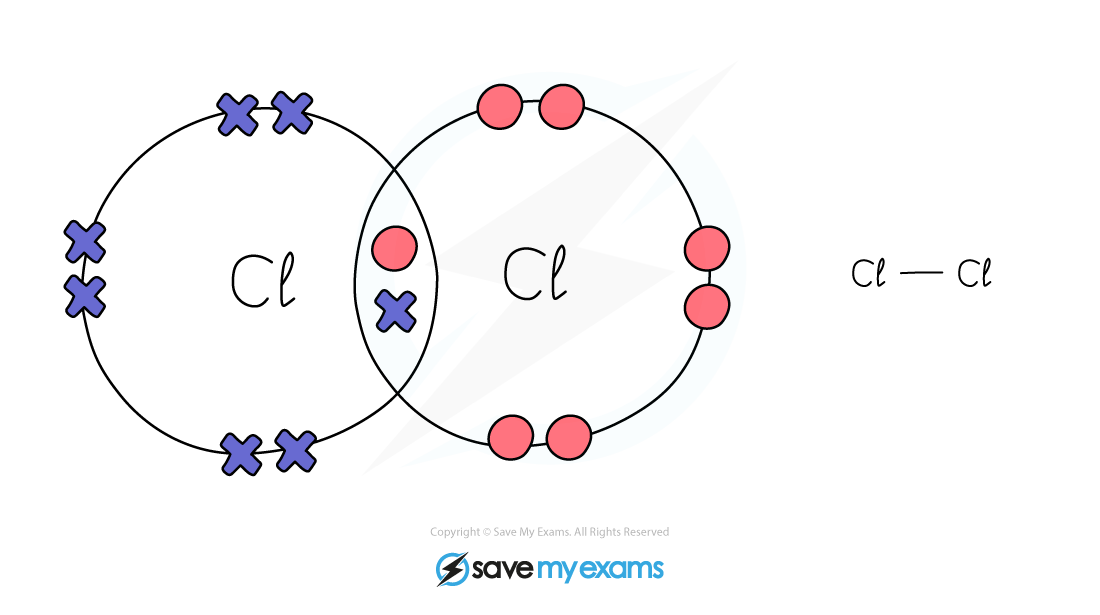
Dot & cross representation of a molecule of chlorine
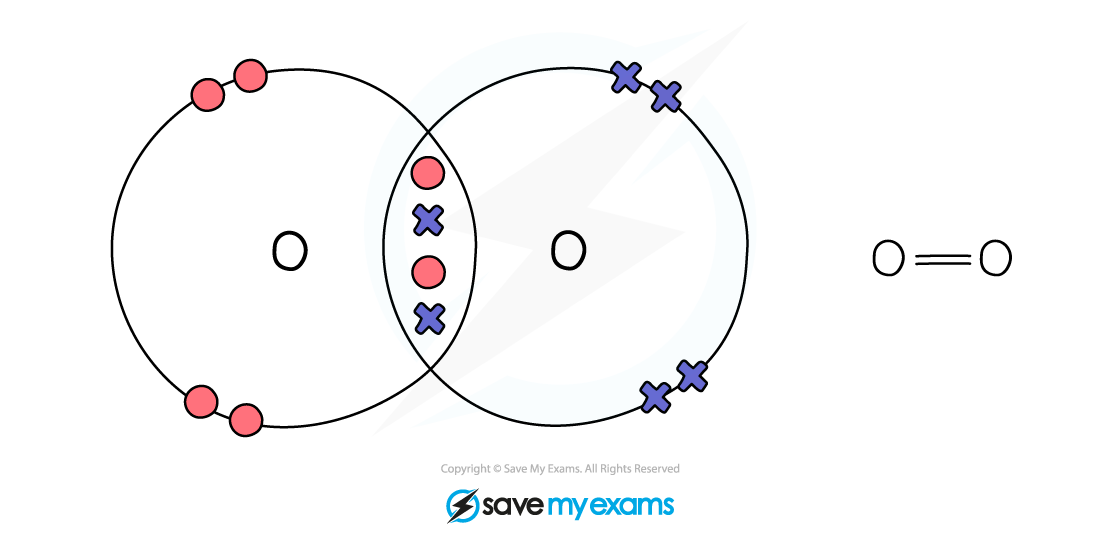
Dot & cross representation of a molecule of oxygen
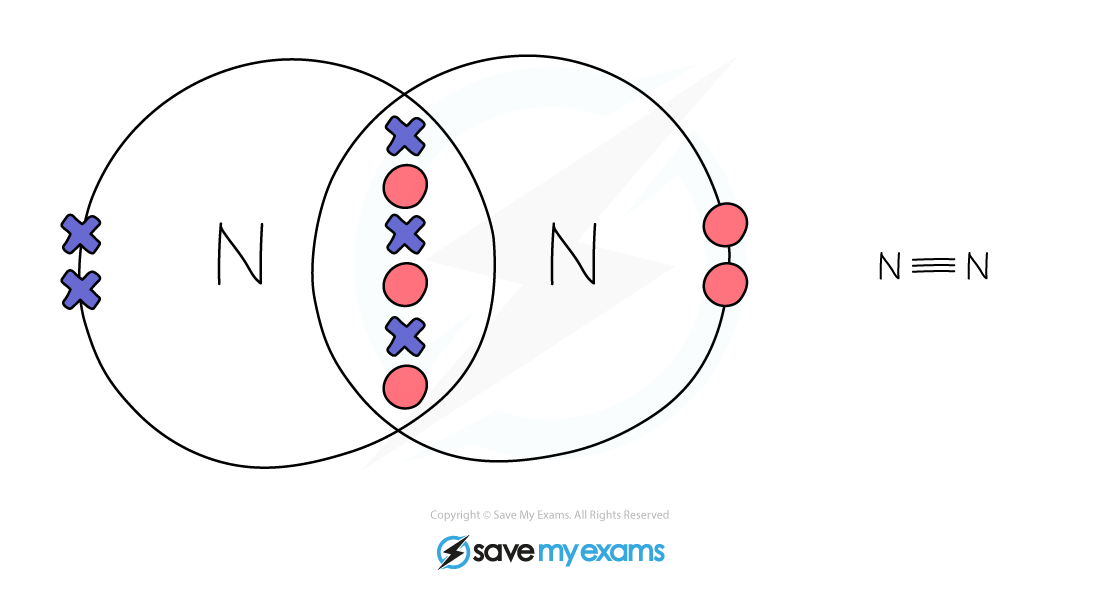
Dot & cross representation of a molecule of nitrogen
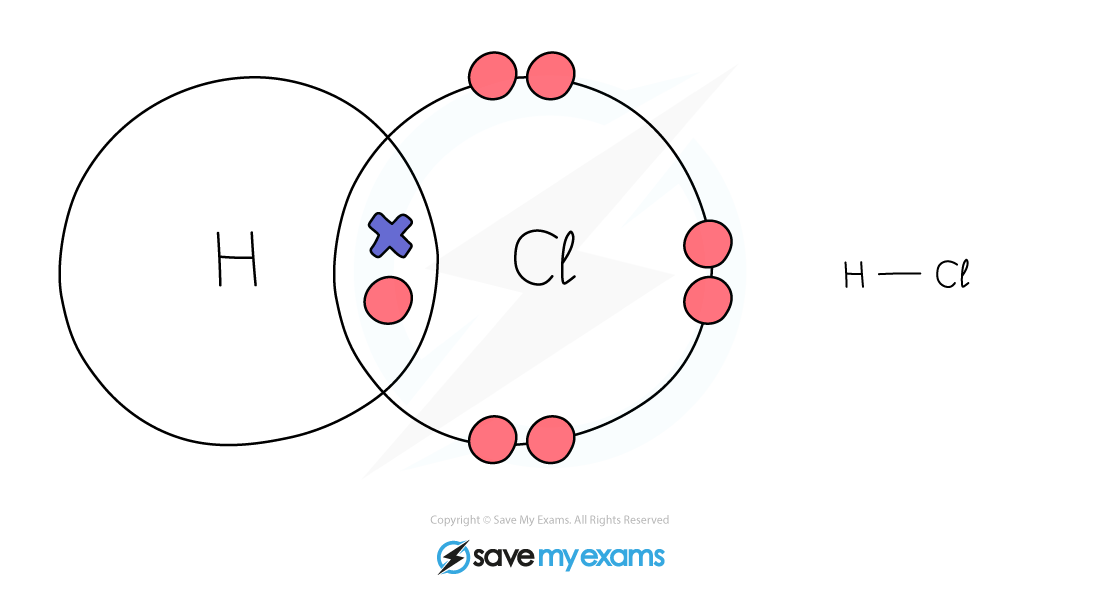
Dot & cross representation of a molecule of hydrogen chloride
Inorganic Molecules
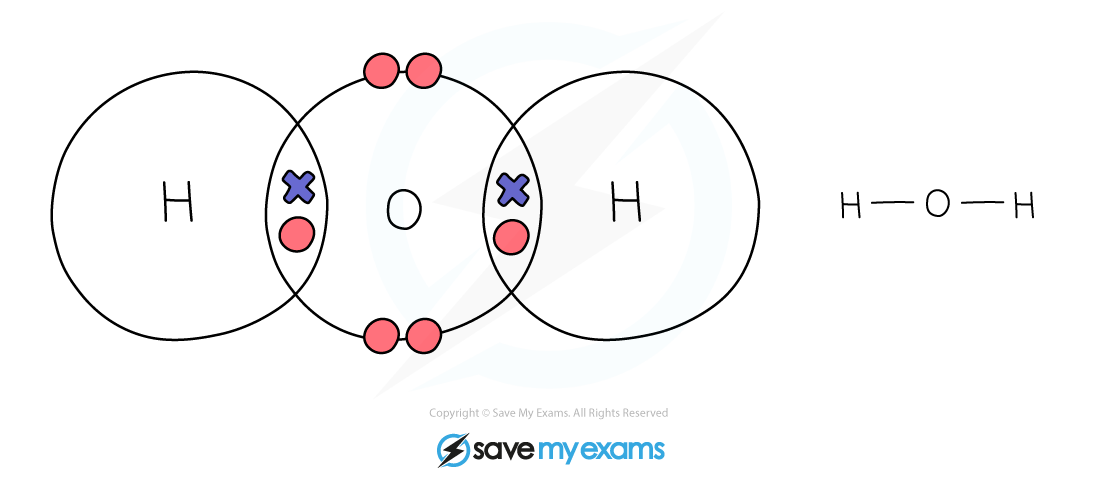
Dot & cross representation of a molecule of water
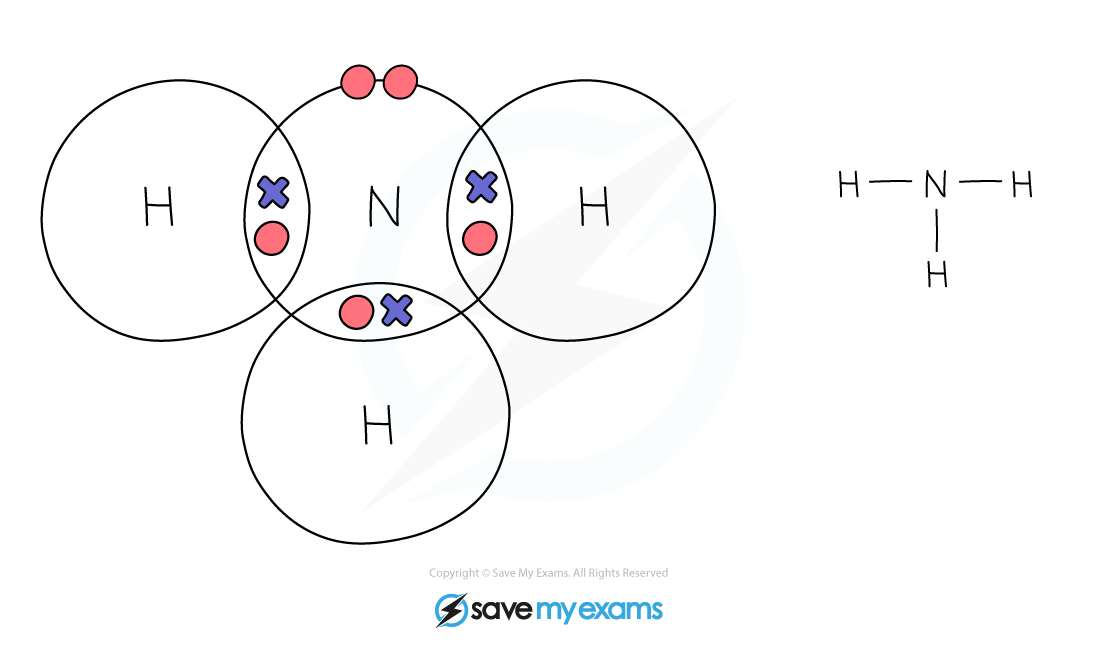
Dot & cross representation of a molecule of ammonia
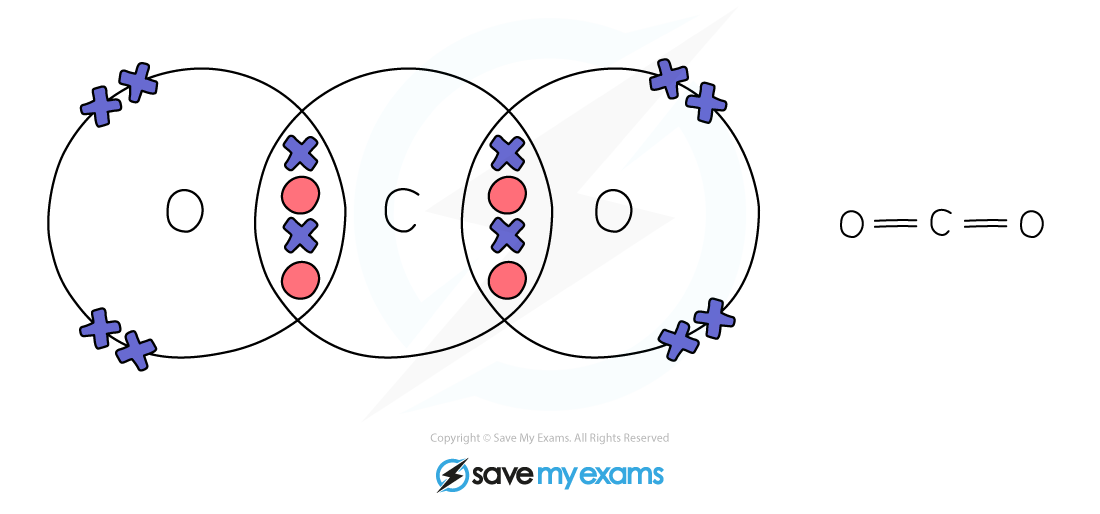
Dot & cross representation of a molecule of carbon dioxide
Organic Molecules
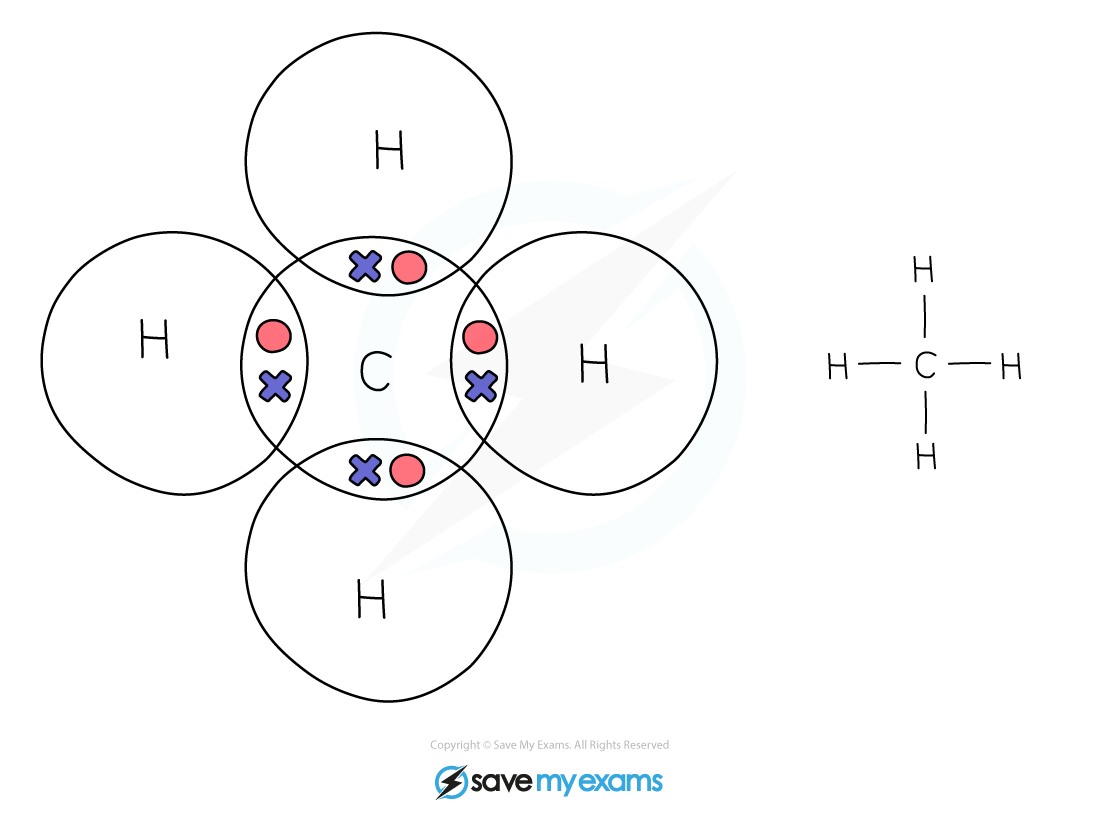
Dot & cross representation of a molecule of methane
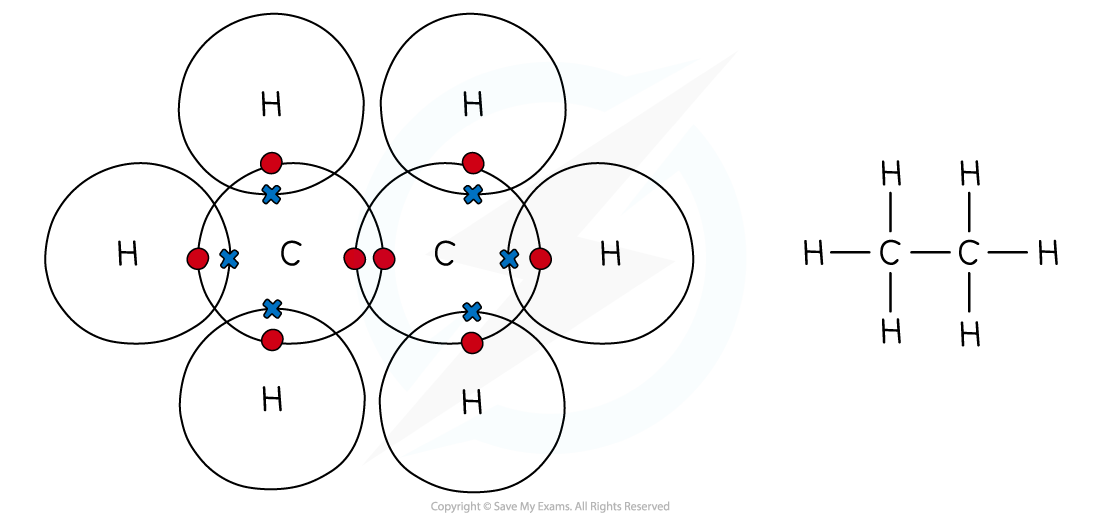
Dot & cross representation of a molecule of ethane
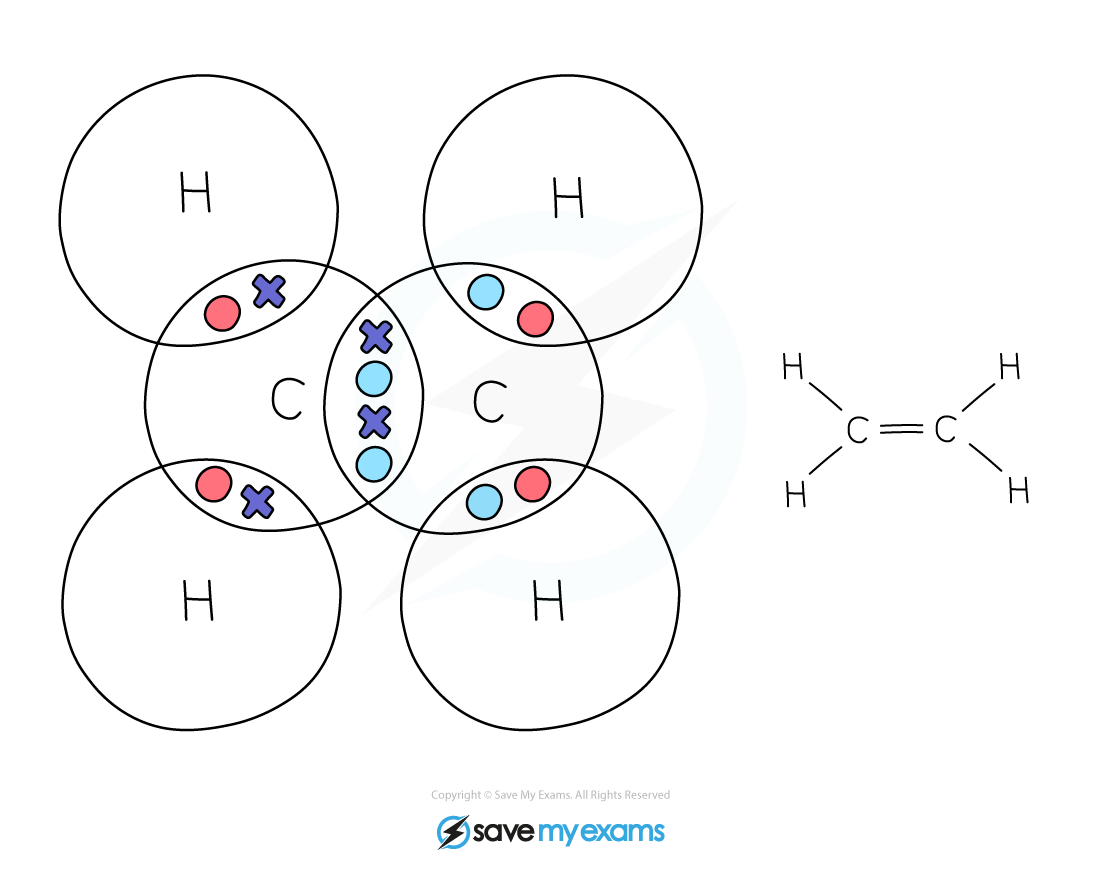
Dot & cross representation of a molecule of ethene
Exam Tip
Simple covalent molecules are small and can be separated into individual molecular units without breaking any chemical bonds (although there will still be strong covalent bonds holding the atoms in each individual molecule together) Giant ionic and covalent structures form huge continuous networks of atoms that are bonded together and cannot be separated into individual units without breaking bonds.
转载自savemyexam

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









