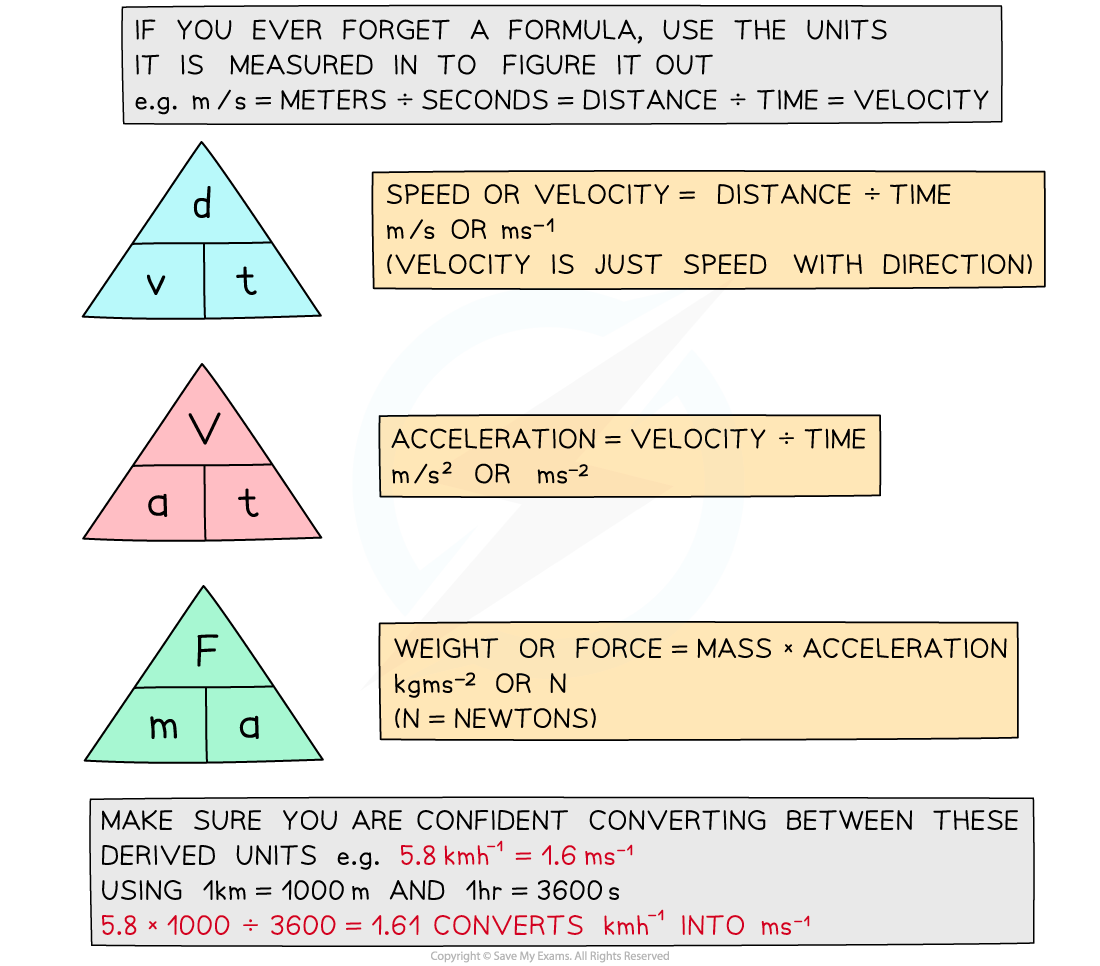
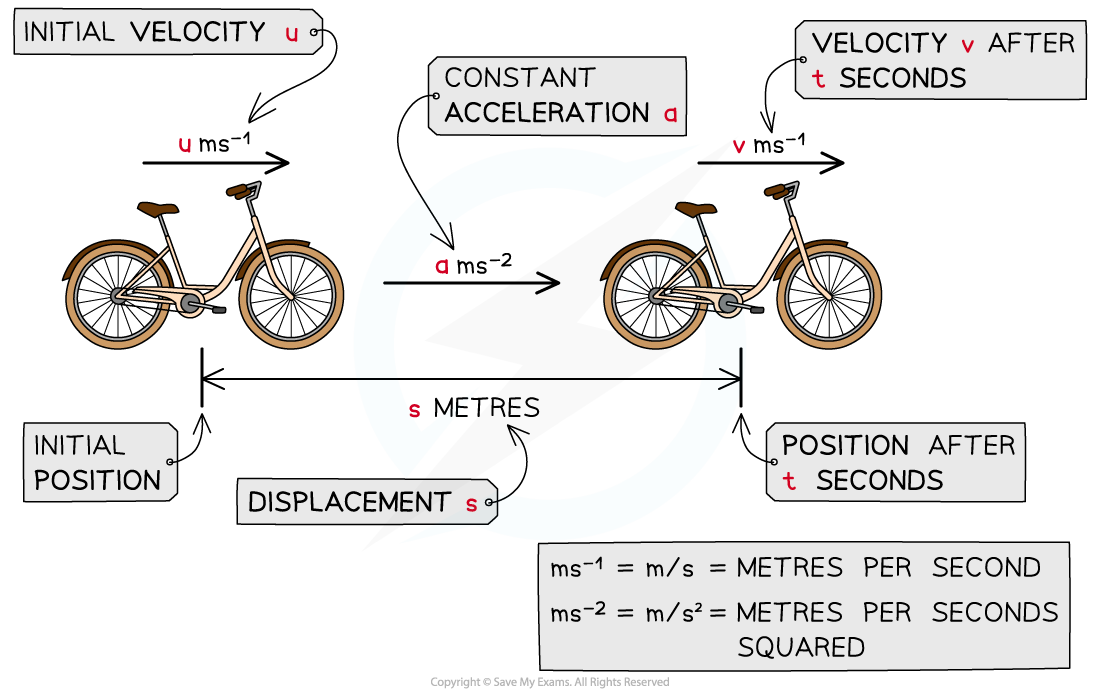
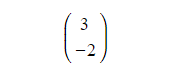AQA A Level Maths: Mechanics复习笔记2.1.1 Displacement-Time Graphs
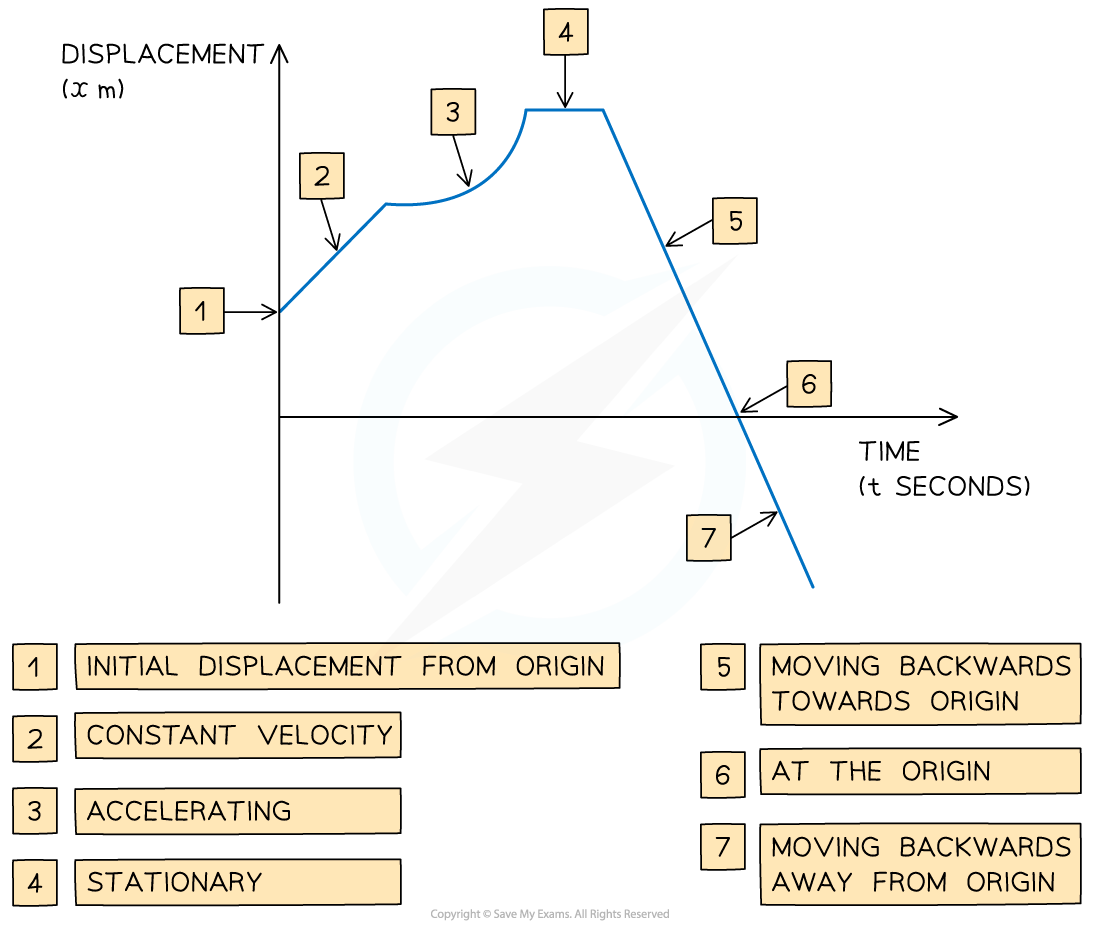
Displacement-Time Graphs What is a displacement-time graph? Displacement-time graphs show the displacement of an object from a fixed origin as it moves in a straight line They show displacem...